Drug-coated stents versus bare metal stents in Academic Research Consortium-defined high bleeding risk patients
- PMID: 32830645
- PMCID: PMC9724924
- DOI: 10.4244/EIJ-D-20-00749
Drug-coated stents versus bare metal stents in Academic Research Consortium-defined high bleeding risk patients
Abstract
Background: More effective and progressively safer generations of drug-elut-ing stents (DES) have replaced bare metal stents (BMS) in rou-tine clinical practice. However, patients considered to be at high bleeding risk (HBR) have traditionally been underrepresented in pivotal DES trials.
Aims: The aim of this study was to model the safety and effectiveness of drug-coated stents (DCS) versus BMS in HBR patients according to the Academic Research Consortium (ARC) criteria.
Methods: Participants from the LEADERS FREE (LF) and LEADERS FREE II (LFII) studies were pooled into one data set. Participants were treated with 30 days of DAPT. The primary safety (composite of cardiac death, myocardial infarction, or stent thrombosis) and effectiveness (target lesion revascularisation) endpoints were compared between DCS and BMS in the subgroup of patients satisfying the ARC-HBR definition using propensity-score modelling.
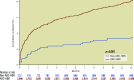
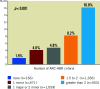
Results: From the 3,635 participants included in the combined LF and LFII data set, 2,898 (79.7%) satisfied the ARC-HBR criteria (DCS: 1,923; BMS: 975). The primary safety endpoint occurred in 184 (9.8%) and in 132 (13.8%) participants in the DCS and BMS groups, respectively (adjusted HR 0.72, 95% CI: 0.57-0.91; p=0.006). The risk of the primary effectiveness endpoint was also significantly lower with DCS (6.2%) versus BMS (8.8%) (adjusted HR 0.70, 95% CI: 0.52-0.94; p=0.016). The safety and effectiveness of DCS versus BMS were consistent according to ARC-HBR status (p for interaction=0.206 and 0.260, respectively).
Conclusions: DCS are safer and more effective than BMS in an ARC-defined HBR population.
Conflict of interest statement
G. Marquis-Gravel declares personal fees from Novartis, Servier, JAMP Pharma, Amgen, Alliance BMS-Pfizer, Canadian Heart Research Center (outside the submitted work); and research funding from Bayer, Duke Clinical Research Institute, Université de Montréal, Montreal Heart Institute Foundation, Fonds de Recherche du Québec - Santé (FRQS), and the Canadian Institutes of Health Research (CIHR). P. Urban reports personal fees from Biosensors and Edwards Lifesciences, outside the submitted work, and other from CERC and MedAlliance. S. Copt, S.S. Slama, and H.P. Stoll are full-time paid employees of Biosensors. D. Capodanno and S.J. Pocock report personal fees from Biosensors (outside the submitted work). J.F. Tanguay reports grants from the Duke Clinical Research Institute during the conduct of the study, grants and other from Abbott Vascular, Bayer, and Biosensors, other from BMS-Pfizer Alliance, and grants and other from Novartis outside the submitted work. R. Mehran reports grants, personal fees and other from Abbott Laboratories, grants and other from Abiomed and Bristol-Myers Squibb, grants and personal fees from Chiesi, grants from Applied Therapeutics, AstraZeneca, Bayer, Beth Israel Deaconess, CERC, Concept Medical, CSL Behring, DSI, Medtronic, Novartis Pharmaceuticals, and OrbusNeich, other from Merck, The Medicines Company, Spectranetics/Philips/Volcano Corp, Watermark Research Partners, Claret Medical, and Elixir Medical, personal fees from Boston Scientific, CardiaWave, Janssen Scientific Affairs, Medscape/WebMD, Roivant Services, Sanofi, Siemens Medical Solutions, Medtelligence (Janssen Scientific Affairs), ACC, and AMA, outside the submitted work, and non-financial support and other from Idorsia Pharmaceuticals Ltd., and Regeneron Pharmaceuticals. M.C. Morice is a shareholder and the CEO of CERC, one of the contract research organisations conducting LF and LFII. M.W. Krucoff reports grants and personal fees from Biosensors (during the conduct of the study), and from Abbott Vascular, Medtronic, OrbusNeich, Terumo, and Cordis/Johnson & Johnson (outside the submitted work). The other authors have no conflicts of interest to declare. The Guest Editor reports lecture fees paid to his institution from Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis, consultancy fees paid to his institution from Boehringer Ingelheim, and grant support from Bayer Healthcare, Boston Scientific, Biotronik, Edwards Lifesciences, GlaxoSmithKline, Medtronic, and Pfizer.
Figures



References
-
- Piccolo R, Bonaa KH, Efthimiou O, Varenne O, Baldo A, Urban P, Kaiser C, Remkes W, Räber L, de Belder A, van ’t Hof AWJ, Stankovic G, Lemos PA, Wilsgaard T, Reifart J, Rodríguez AE, Ribeiro EE, Serruys PWJC, Abizaid A, Sabate M, Byrne RA, de la Torre Hernandez JM, Wijns W, Jüni P, Windecker S, Valgimigli M Coronary Stent Trialists’ Collaboration. Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet. 2019;393:2503–10. - PubMed
-
- Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrié D, Naber C, Lipiecki J, Richardt G, Iñiguez A, Brunel P, Valdes-Chavarri M, Garot P, Talwar S, Berland J, Abdellaoui M, Eberli F, Oldroyd K, Zambahari R, Gregson J, Greene S, Stoll HP, Morice MC LEADERS FREE Investigators. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N Engl J Med. 2015;373:2038–47. - PubMed
-
- Windecker S, Latib A, Kedhi E, Kirtane AJ, Kandzari DE, Mehran R, Price MJ, Abizaid A, Simon DI, Worthley SG, Zaman A, Hudec M, Poliacikova P, Abdul Ghapar AKB, Selvaraj K, Petrov I, Mylotte D, Pinar E, Moreno R, Fabbiocchi F, Pasupati S, Kim HS, Aminian A, Tie C, Wlodarczak A, Hur SH, Marx SO, Jankovic I, Brar S, Bousquette L, Liu M, Stone GW ONYX ONE Investigators. Polymer-based or Polymer-free Stents in Patients at High Bleeding Risk. N Engl J Med. 2020;382:1208–18. - PubMed
-
- Krucoff M, Urban P, Tanguay JF, McAndrew T, Zhang Y, Rao SV, Morice MC, Price MJ, Cohen DJ, Abdel-Wahab M, Mehta SR, Faurie B, McLaurin B, Diaz C, Stoll HP, Pocock S, Leon MB. A Global Approach to High Bleeding Risk Patients with Polymer-Free Drug-Coated Coronary Stents: The LF II Study. Circ Cardiovasc Interv. 2020;13:e008603. doi: 10.1161/CIRCINTERVENTIONS.119.008603. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

