Canakinumab to reduce deterioration of cardiac and respiratory function in SARS-CoV-2 associated myocardial injury with heightened inflammation (canakinumab in Covid-19 cardiac injury: The three C study)
- PMID: 32830894
- PMCID: PMC7461303
- DOI: 10.1002/clc.23451
Canakinumab to reduce deterioration of cardiac and respiratory function in SARS-CoV-2 associated myocardial injury with heightened inflammation (canakinumab in Covid-19 cardiac injury: The three C study)
Abstract
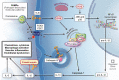
Background: In patients with Covid-19, myocardial injury and increased inflammation are associated with morbidity and mortality. We designed a proof-of-concept randomized controlled trial to evaluate whether treatment with canakinumab prevents progressive respiratory failure and worsening cardiac dysfunction in patients with SARS-CoV2 infection, myocardial injury, and high levels of inflammation.
Hypothesis: The primary hypothesis is that canakiumab will shorten time to recovery.
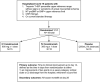
Methods: The three C study (canakinumab in Covid-19 Cardiac Injury, NCT04365153) is a double-blind, randomized controlled trial comparing canakinumab 300 mg IV, 600 mg IV, or placebo in a 1:1:1 ratio in hospitalized Covid-19 patients with elevations in troponin and C-reactive protein (CRP). The primary endpoint is defined as the time in days from randomization to either an improvement of two points on a seven category ordinal scale or discharge from the hospital, whichever occurs first up to 14 days postrandomization. The secondary endpoint is mortality at day 28. A total of 45 patients will be enrolled with an anticipated 5 month follow up period.
Results: Baseline characteristics for the first 20 randomized patients reveal a predominantly male (75%), elderly population (median 67 years) with a high prevalence of hypertension (80%) and hyperlipidemia (75%). CRPs have been markedly elevated (median 16.2 mg/dL) with modest elevations in high-sensitivity troponin T (median 21 ng/L), in keeping with the concept of enrolling patients with early myocardial injury.
Conclusions: The three C study will provide insights regarding whether IL-1β inhibition may improve outcomes in patients with SARS-CoV2 associated myocardial injury and increased inflammation.
Keywords: Covid-19; SARS-CoV-2; canakinumab; myocardial injury.
© 2020 The Authors. Clinical Cardiology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures
References
-
- World Health Organization . Coronavirus disease 2019 (COVID‐19): situation report, 72 2020.
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1242. - PubMed
-
- Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. 2020. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

