Short-course Benznidazole treatment to reduce Trypanosoma cruzi parasitic load in women of reproductive age (BETTY): a non-inferiority randomized controlled trial study protocol
- PMID: 32831069
- PMCID: PMC7446054
- DOI: 10.1186/s12978-020-00972-1
Short-course Benznidazole treatment to reduce Trypanosoma cruzi parasitic load in women of reproductive age (BETTY): a non-inferiority randomized controlled trial study protocol
Abstract
Background: Retrospective observational studies suggest that transmission of Trypanosoma cruzi does not occur in treated women when pregnant later in life. The level of parasitemia is a known risk factor for congenital transmission. Benznidazole (BZN) is the drug of choice for preconceptional treatment to reduce parasitic load. The fear of treatment-related side effects limits the implementation of the Argentine guideline recommending BZN 60d/300 mg (or equivalent) treatment of T. cruzi seropositive women during the postpartum period to prevent transmission in a future pregnancy. A short and low dose BZN treatment might reduce major side effects and increase compliance, but its efficacy to reduce T. cruzi parasitic load compared to the standard 60d/300 mg course is not yet established. Clinical trials testing alternative BZN courses among women of reproductive age are urgently needed.
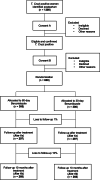
Methods and design: We are proposing to perform a double-blinded, non-inferiority randomized controlled trial comparing a short low dose 30-day treatment with BZN 150 mg/day (30d/150 mg) vs. BZN 60d/300 mg. We will recruit not previously treated T. cruzi seropositive women with a live birth during the postpartum period in Argentina, randomize them at 6 months postpartum, and follow them up with the following specific aims: Specific aim 1: to measure the effect of BZN 30d/150 mg compared to 60d/300 mg preconceptional treatment on parasitic load measured by the frequency of positive Polymerase Chain Reaction (PCR) (primary outcome) and by real-time quantitative PCR (qPCR), immediately and 10 months after treatment. Specific aim 2: to measure the frequency of serious adverse events and/or any adverse event leading to treatment interruption.
Trial registration: ClinicalTrials.gov . Identifier: NCT03672487 . Registered 14 September 2018.
Antecedentes: Estudios observacionales retrospectivos sugieren que la transmisión del Trypanosoma cruzi no ocurre en mujeres tratadas cuando quedan embarazadas más adelante. La carga parasitaria en la sangre es un factor de riesgo conocido para la transmisión congénita. El Benznidazol (BZN) es el fármaco de elección para el tratamiento preconcepcional de la mujer en edad fértil para reducir la carga parasitaria.
El temor a los efectos adversos relacionados con el tratamiento limita la implementación de la Guía Argentina que recomienda el tratamiento con BZN 60d/300 mg (o equivalente) en mujeres seropositivas a T. cruzi durante el período posparto para prevenir la transmisión en un futuro embarazo. Un tratamiento más corto y con una dosis menor de BZN podría reducir los eventos adversos y mejorar la adherencia al mismo, pero aún no se ha establecido su eficacia para reducir la carga parasitaria de T. cruzi en comparación con el esquema estándar de 60d/300 mg. Se necesitan con urgencia ensayos clínicos que prueben esquemas alternativos de BZN en mujeres en edad reproductiva.
Métodos y diseño: Estamos proponiendo realizar un ensayo clínico controlado aleatorizado doble ciego de no inferioridad que compara un tratamiento corto de 30 días a dosis bajas con BZN 150 mg/día (30d/150 mg) versus BZN 60d/300 mg. Se reclutarán mujeres seropositivas para infección por T. cruzi no tratadas previamente, que hayan tenido un nacido vivo, durante el periodo de posparto en Argentina. Las mujeres serán aleatorizadas a los seis meses posparto y serán seguidas, con los siguientes objetivos específicos:
Objetivo específico 1: evaluar el efecto del tratamiento preconcepcional con BZN 30d/150 mg comparado con BZN 60d/300 mg por medio de la carga parasitaria, medida por la frecuencia de PCR positiva (resultado primario) y PCR cuantitativa a tiempo real (qPCR), al final del tratamiento y a los 10 meses post tratamiento.
Objetivo específico 2: medir la frecuencia de eventos adversos serios y/o cualquier otro evento adverso que conduzcan a la interrupción del tratamiento con BZN 30d / 150 mg en comparación con BZN 60d/300 mg.
Keywords: Benznidazole; Chagas disease; Preconception care; Randomized controlled trial; Trypanosoma cruzi.
Conflict of interest statement
The authors declare that they have no competing interests.
References
-
- Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. 2015;90(6):33–43. - PubMed
-
- Yadon ZE, Schmunis GA. Congenital Chagas disease: estimating the potential risk in the United States. Am J Trop Med Hyg. 2009;81(6):927–933. - PubMed
-
- Buekens P, Almendares O, Carlier Y, Dumonteil E, Eberhard M, Gamboa-Leon R, et al. Mother-to-child transmission of Chagas' disease in North America: why don't we do more? Matern Child Health J. 2008;12(3):283–286. - PubMed
-
- Carlier Y, Truyens C. Congenital Chagas disease as an ecological model of interactions between Trypanosoma cruzi parasites, pregnant women, placenta and fetuses. Acta Trop. 2015;151:103–115. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical