Artesunate Activation by Heme in an Aqueous Medium
- PMID: 32831389
- PMCID: PMC7442224
- DOI: 10.1016/j.ica.2019.119029
Artesunate Activation by Heme in an Aqueous Medium
Abstract
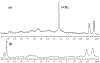
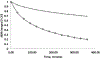
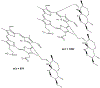
The reaction between the antimalarial drug artesunate (ATS) and ferriprotoporphyrin_(IX) (FPIX) in the presence of glutathione (GSH) has been monitored by nuclear magnetic resonance (NMR) spectroscopy. By following the disappearance of resonances of protons near the endoperoxide group in ATS, the rate at which the drug is activated can be directly measured. In an aqueous medium, the rate of ATS activation is limited by the rate of reduction of the FPIX Fe(III) center by GSH. The reaction is observed to slow dramatically in the presence of other heme binding antimalarial drugs. These findings explain the long observed antagonism between artemisinin derivatives and quinoline-based drugs. This discovery suggests that combination therapy that involves artemisinin or any of its derivatives and a quinoline-based drug may be compromised.
Figures




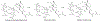


Similar articles
-
Solution structures of antimalarial drug-heme complexes.Biochemistry. 2002 Aug 13;41(32):10245-55. doi: 10.1021/bi020195i. Biochemistry. 2002. PMID: 12162739
-
Structure of the amodiaquine-FPIX mu oxo dimer solution complex at atomic resolution.Inorg Chem. 2004 Dec 13;43(25):8078-84. doi: 10.1021/ic0489948. Inorg Chem. 2004. PMID: 15578847
-
Antimalarial drugs influence the pH dependent solubility of heme via apparent nucleation phenomena.Mol Biochem Parasitol. 2001 Jan 15;112(1):11-7. doi: 10.1016/s0166-6851(00)00342-x. Mol Biochem Parasitol. 2001. PMID: 11166382
-
Ferriprotoporphyrin IX, phospholipids, and the antimalarial actions of quinoline drugs.Life Sci. 2004 Mar 5;74(16):1957-72. doi: 10.1016/j.lfs.2003.10.003. Life Sci. 2004. PMID: 14967191 Review.
-
Antimalarial compounds: from bench to bedside.J Exp Biol. 2003 Nov;206(Pt 21):3753-9. doi: 10.1242/jeb.00653. J Exp Biol. 2003. PMID: 14506210 Review.
Cited by
-
The Role of the Iron Protoporphyrins Heme and Hematin in the Antimalarial Activity of Endoperoxide Drugs.Pharmaceuticals (Basel). 2022 Jan 4;15(1):60. doi: 10.3390/ph15010060. Pharmaceuticals (Basel). 2022. PMID: 35056117 Free PMC article. Review.
-
Artemisinin-Based Drugs Target the Plasmodium falciparum Heme Detoxification Pathway.Antimicrob Agents Chemother. 2021 Mar 18;65(4):e02137-20. doi: 10.1128/AAC.02137-20. Print 2021 Mar 18. Antimicrob Agents Chemother. 2021. PMID: 33495226 Free PMC article.
-
Artesunate regulates the proliferation and differentiation of neural stem cells by activating the JAK‑2/STAT‑3 signaling pathway in ischemic stroke.Exp Ther Med. 2022 Nov 16;25(1):2. doi: 10.3892/etm.2022.11701. eCollection 2023 Jan. Exp Ther Med. 2022. PMID: 36561626 Free PMC article.
References
-
- Tu Y The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nature Medicine 2011, 17, 1217–1220. - PubMed
-
- Ashley EA; Dhorda M; Fairhurst RM; Amaratunga C; Lim P; Suon S; Sreng S; Anderson JM; Mao S; Sam B; Sopha C; Chuor CM; Nguon C; Sovannaroth S; Pukrittayakamee S; Jittamala P; Chotivanich K; Chutasmit K; Suchatsoonthorn C; Runcharoen R; Hien TT; Thuy-Nhien NT; Thanh NV; Phu NH; Htut Y; Han K-T; Aye KH; Mokuolu OA; Olaosebikan RR; Folaranmi OO; Mayxay M; Khanthavong M; Hongvanthong B; Newton PN; Onyamboko MA; Fanello CI; Tshefu AK; Mishra N ; Valecha N; Phyo AP; Nosten F; Yi P; Tripura R; Borrmann S; Bashraheil M; Peshu J; Faiz MA; Ghose A; Hossain MA; Samad R; Rahman MR; Hasan MM; Islam A; Miotto O; Amato R; Maclnnis B; Stalker J; Kwiatkowski DP; Bozdech Z; Jeeyapant A; Cheah PY; Sakulthaew T; Chalk J; Intharabut B; Silamut K; Lee SJ; Vihokhern B; Kunasol C; Imwong M; Taming J; Taylor WJ; Yeung S; Woodrow CJ; Flegg JA; Das D; Smith J; Venkatesan M; Plowe CV; Stepniewska K; Guerin PJ; Dondorp AM; Day NP; White NJ Spread of artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2014, 371, 411–423. - PMC - PubMed
-
- Amaratunga C; Lim P; Suon S; Sreng S; Mao S; Sopha C; Sam B; Dek D; Try V; Amato R; Blessborn D; Song L; Tullo GS, Fay MP; Anderson JM; Tarning J; Fairhurst RM Dihydroartemisinin–piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. The Lancet Infectious Diseases 2016, 16, 357–365. - PMC - PubMed
-
- Dondorp AM; Nosten F; Yi P; Das D; Phyo AP; Tarning J; Lwin KM; Ariey F Hanpithakpong W; Lee SJ; Ringwald P; Silamut K; Imwong M; Chotivanich K; Lim P; Herdman T; An SS; Yeung S; Singhasivanon P; Day NP; Lindegardh N; Socheat D; White NJ Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2009, 361, 455–467. Erratum in: N. Engl. J. Med. 361, 1714. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
