Prevention of Oxygen-Induced Inflammatory Lung Injury by Caffeine in Neonatal Rats
- PMID: 32831996
- PMCID: PMC7429812
- DOI: 10.1155/2020/3840124
Prevention of Oxygen-Induced Inflammatory Lung Injury by Caffeine in Neonatal Rats
Abstract
Background: Preterm birth implies an array of respiratory diseases including apnea of prematurity and bronchopulmonary dysplasia (BPD). Caffeine has been introduced to treat apneas but also appears to reduce rates of BPD. Oxygen is essential when treating preterm infants with respiratory problems but high oxygen exposure aggravates BPD. This experimental study is aimed at investigating the action of caffeine on inflammatory response and cell death in pulmonary tissue in a hyperoxia-based model of BPD in the newborn rat. Material/Methods. Lung injury was induced by hyperoxic exposure with 80% oxygen for three (P3) or five (P5) postnatal days with or without recovery in ambient air until postnatal day 15 (P15). Newborn Wistar rats were treated with PBS or caffeine (10 mg/kg) every two days beginning at the day of birth. The effects of caffeine on hyperoxic-induced pulmonary inflammatory response were examined at P3 and P5 immediately after oxygen exposure or after recovery in ambient air (P15) by immunohistological staining and analysis of lung homogenates by ELISA and qPCR.
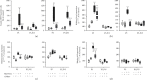
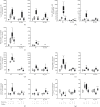
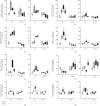
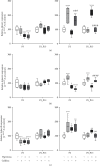
Results: Treatment with caffeine significantly attenuated changes in hyperoxia-induced cell death and apoptosis-associated factors. There was a significant decrease in proinflammatory mediators and redox-sensitive transcription factor NFκB in the hyperoxia-exposed lung tissue of the caffeine-treated group compared to the nontreated group. Moreover, treatment with caffeine under hyperoxia modulated the transcription of the adenosine receptor (Adora)1. Caffeine induced pulmonary chemokine and cytokine transcription followed by immune cell infiltration of alveolar macrophages as well as increased adenosine receptor (Adora1, 2a, and 2b) expression.
Conclusions: The present study investigating the impact of caffeine on the inflammatory response, pulmonary cell degeneration and modulation of adenosine receptor expression, provides further evidence that caffeine acts as an antioxidative and anti-inflammatory drug for experimental oxygen-mediated lung injury. Experimental studies may broaden the understanding of therapeutic use of caffeine in modulating detrimental mechanisms involved in BPD development.
Copyright © 2020 Stefanie Endesfelder et al.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures


References
-
- Negi R., Pande D., Kumar A., Khanna R. S., Khanna H. D. Evaluation of biomarkers of oxidative stress and antioxidant capacity in the cord blood of preterm low birth weight neonates. The Journal of Maternal-Fetal & Neonatal Medicine. 2012;25(8):1338–1341. doi: 10.3109/14767058.2011.633672. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

