Novel Blocker of Onco SK3 Channels Derived from Scorpion Toxin Tamapin and Active against Migration of Cancer Cells
- PMID: 32832033
- PMCID: PMC7429966
- DOI: 10.1021/acsmedchemlett.0c00300
Novel Blocker of Onco SK3 Channels Derived from Scorpion Toxin Tamapin and Active against Migration of Cancer Cells
Abstract
Peptide-based therapy against cancer is a field of great interest for biomedical developments. Since it was shown that SK3 channels promote cancer cell migration and metastatic development, we started using these channels as targets for the development of antimetastatic drugs. Particularly, tamapin (a peptide found in the venom of the scorpion Mesobuthus tamulus) is the most specific toxin against the SK2 channel currently known. Considering this fact, we designed diverse tamapin mutants based on three different hypotheses to discover a new potent molecule to block SK3 channels. We performed in vitro studies to evaluate this new toxin derivative inhibitor of cancer cell migration. Our results can be used to generate a new tamapin-based therapy against cancer cells that express SK3 channels.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



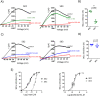
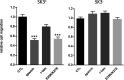
Similar articles
-
Tamapin, a venom peptide from the Indian red scorpion (Mesobuthus tamulus) that targets small conductance Ca2+-activated K+ channels and afterhyperpolarization currents in central neurons.J Biol Chem. 2002 Nov 29;277(48):46101-9. doi: 10.1074/jbc.M206465200. Epub 2002 Sep 17. J Biol Chem. 2002. PMID: 12239213
-
Cytotoxicity of recombinant tamapin and related toxin-like peptides on model cell lines.Chem Res Toxicol. 2014 Jun 16;27(6):960-7. doi: 10.1021/tx4004193. Epub 2014 May 12. Chem Res Toxicol. 2014. PMID: 24821061
-
New alkyl-lipid blockers of SK3 channels reduce cancer cell migration and occurrence of metastasis.Curr Cancer Drug Targets. 2011 Nov;11(9):1111-25. doi: 10.2174/156800911798073069. Curr Cancer Drug Targets. 2011. PMID: 21999627
-
Small conductance Ca2+-activated K+ channels as targets of CNS drug development.Curr Drug Targets CNS Neurol Disord. 2004 Jun;3(3):161-7. doi: 10.2174/1568007043337472. Curr Drug Targets CNS Neurol Disord. 2004. PMID: 15180477 Review.
-
Diverse Structural Features of Potassium Channels Characterized by Scorpion Toxins as Molecular Probes.Molecules. 2019 May 29;24(11):2045. doi: 10.3390/molecules24112045. Molecules. 2019. PMID: 31146335 Free PMC article. Review.
Cited by
-
Potassium channel-driven bioelectric signalling regulates metastasis in triple-negative breast cancer.EBioMedicine. 2022 Jan;75:103767. doi: 10.1016/j.ebiom.2021.103767. Epub 2021 Dec 18. EBioMedicine. 2022. PMID: 34933180 Free PMC article.
-
A Deep Graph Network-Enhanced Sampling Approach to Efficiently Explore the Space of Reduced Representations of Proteins.Front Mol Biosci. 2021 Apr 29;8:637396. doi: 10.3389/fmolb.2021.637396. eCollection 2021. Front Mol Biosci. 2021. PMID: 33996896 Free PMC article.
-
Endogenous ether lipids differentially promote tumor aggressiveness by regulating the SK3 channel.J Lipid Res. 2024 May;65(5):100544. doi: 10.1016/j.jlr.2024.100544. Epub 2024 Apr 18. J Lipid Res. 2024. PMID: 38642894 Free PMC article.
-
Modifications of Plasma Membrane Organization in Cancer Cells for Targeted Therapy.Molecules. 2021 Mar 25;26(7):1850. doi: 10.3390/molecules26071850. Molecules. 2021. PMID: 33806009 Free PMC article. Review.
-
Antiprotozoal Activity Against Entamoeba hystolitica and Giardia lamblia of Cyclopeptides Isolated from Annona diversifolia Saff.Molecules. 2024 Nov 28;29(23):5636. doi: 10.3390/molecules29235636. Molecules. 2024. PMID: 39683795 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Chemical Information

