Visualizing semipermeability of the cell membrane using a pH-responsive ratiometric AIEgen
- PMID: 32832051
- PMCID: PMC7422962
- DOI: 10.1039/d0sc02097d
Visualizing semipermeability of the cell membrane using a pH-responsive ratiometric AIEgen
Abstract
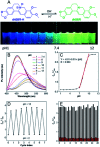
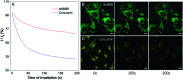
In clinical chemotherapy, some basic drugs cannot enter the hydrophobic cell membrane because of ionization in the acidic tumor microenvironment, a phenomenon known as ion trapping. In this study, we developed a method to visualize this ion trapping phenomenon by utilizing a pH-responsive ratiometric AIEgen, dihydro berberine (dhBBR). By observing the intracellular fluorescence of dhBBR, we found that non-ionized dhBBR can enter cells more easily than ionized forms, which is in accordance with the concept of ion trapping. In addition, dhBBR shows superior anti-photobleaching ability to Curcumin thanks to its AIE properties. These results suggest that dhBBR can serve as a bioprobe for ion trapping.
This journal is © The Royal Society of Chemistry 2020.
Figures



Similar articles
-
Berberine derivatives reduce atherosclerotic plaque size and vulnerability in apoE(-/-) mice.J Transl Med. 2014 Nov 26;12:326. doi: 10.1186/s12967-014-0326-7. J Transl Med. 2014. PMID: 25425200 Free PMC article.
-
Transforming berberine into its intestine-absorbable form by the gut microbiota.Sci Rep. 2015 Jul 15;5:12155. doi: 10.1038/srep12155. Sci Rep. 2015. PMID: 26174047 Free PMC article.
-
An acidic pH independent piperazine-TPE AIEgen as a unique bioprobe for lysosome tracing.Chem Sci. 2017 Nov 1;8(11):7593-7603. doi: 10.1039/c7sc03515b. Epub 2017 Sep 18. Chem Sci. 2017. PMID: 29568423 Free PMC article.
-
Aggregation-Induced Emission Luminogens for Activity-Based Sensing.Acc Chem Res. 2019 Sep 17;52(9):2559-2570. doi: 10.1021/acs.accounts.9b00305. Epub 2019 Aug 22. Acc Chem Res. 2019. PMID: 31436083 Review.
-
Designs and Applications of Multi-stimuli Responsive FRET Processes in AIEgen-Functionalized and Bi-fluorophoric Supramolecular Materials.Top Curr Chem (Cham). 2022 Dec 10;381(1):2. doi: 10.1007/s41061-022-00412-7. Top Curr Chem (Cham). 2022. PMID: 36495421 Review.
Cited by
-
The complex relationship between multiple drug resistance and the tumor pH gradient: a review.Cancer Drug Resist. 2022 Apr 3;5(2):277-303. doi: 10.20517/cdr.2021.134. eCollection 2022. Cancer Drug Resist. 2022. PMID: 35800371 Free PMC article. Review.
-
Synthesis of Berberine and Canagliflozin Chimera and Investigation into New Antibacterial Activity and Mechanisms.Molecules. 2022 May 5;27(9):2948. doi: 10.3390/molecules27092948. Molecules. 2022. PMID: 35566298 Free PMC article.
-
The Bioimaging Story of AIEgens.Chem Biomed Imaging. 2023 Jul 3;1(6):509-521. doi: 10.1021/cbmi.3c00056. eCollection 2023 Sep 25. Chem Biomed Imaging. 2023. PMID: 39473571 Free PMC article. Review.
-
BioAIEgens derived from rosin: how does molecular motion affect their photophysical processes in solid state?Nat Commun. 2021 Mar 19;12(1):1773. doi: 10.1038/s41467-021-22061-y. Nat Commun. 2021. PMID: 33741995 Free PMC article.
References
-
- Marrack P., Lo D., Brinster R., Palmiter R., Burkly L., Flavell R. H., Kappler J. Cell. 1988;53:627–634. - PubMed
-
- Sutherland R. Science. 1988;240:177–184. - PubMed
-
- Simons K., Ikonen E. Nature. 1997;387:569–572. - PubMed
-
- Singer S. J., Nicolson G. L. Science. 1972;175:720–731. - PubMed
-
- Raghunand N., Mahoney B. P., Gillies R. J. Biochem. Pharmacol. 2003;66:1219–1229. - PubMed
LinkOut - more resources
Full Text Sources

