Problem-solving therapy rather than treatment as usual for adults after self-harm: a pragmatic, feasibility, randomised controlled trial (the MIDSHIPS trial)
- PMID: 32832098
- PMCID: PMC7437039
- DOI: 10.1186/s40814-020-00668-0
Problem-solving therapy rather than treatment as usual for adults after self-harm: a pragmatic, feasibility, randomised controlled trial (the MIDSHIPS trial)
Abstract
Background: Non-fatal self-harm is one of the commonest reasons for adults' emergency hospital attendance. Although strongly associated with fatal and non-fatal repetition, there is weak evidence about effective interventions-and no clear NICE guidance or clinical consensus concerning aftercare. We examined the practicability of a definitive trial to evaluate problem-solving therapy (PST) to reduce repetition of self-harm; MIDSHIPS is a single-centre, parallel-group, individually randomised controlled feasibility trial comparing treatment-as-usual (TAU) alone to TAU plus up to six sessions of brief problem-solving therapy (PST) with adults who had recently attended hospital because of self-harm. Objectives were to adapt the intervention for a UK setting, train therapists, recruit and randomise patients, deliver PST under supervision, and establish comparative outcomes, assessed blindly.
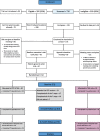
Methods: We adapted the problem-solving intervention from an earlier trial and trained a mental-health nurse to deliver it. Adult patients attending the general hospital for self-harm were recruited while undergoing psychosocial assessment by the mental health team, and 62 were randomly allocated (32 TAU, 30 PST). The primary outcome assessed repeat hospital attendance due to further self-harm 6 months post-randomisation. Secondary outcomes included participant-reported outcomes and service use at 3 and 6 months post-randomisation.
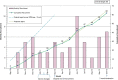
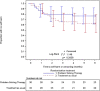
Results: The recruitment period had to be extended and 710 patients screened in order to establish a trial sample of the planned size (N = 62). A quarter of participants allocated to PST did not undertake the therapy offered; those who received PST attended a median of three sessions. Secondary outcomes were established for 49 (79%) participants at 6 months; all participants' hospital records were retrieved. Repetition of self-harm leading to hospital presentation occurred in 19 of the 62 participants (30.6%, 95% CI 19.2%, 42.1%) within 6 months of randomisation. Promising differential rates of self-harm were observed with an event rate of 23.3% (95% CI 8.2%, 38.5%) in the PST arm; and 37.5% (95% CI 20.7%, 54.3%) in TAU. Economic findings were also encouraging, with a small QALY gain (0.0203) in the PST arm together with less reported use of the NHS in the PST arm (average £2120) than with TAU-only (£2878).
Conclusions: The feasibility trial achieved its objectives despite considerable difficulties with recruitment-adapting the PST, training a therapist, recruiting patients who had recently self-harmed, delivering the therapy, and establishing primary and secondary outcomes. These data provide a robust platform for a definitive multicentre randomised controlled trial of brief problem-solving therapy after hospital attendance due to self-harm.
Trial registration: Identification number and URL: ISRCTN54036115 http://www.isrctn.com/search?q=midships. Registered: 13 January 2012.
Keywords: Adults; Feasibility RCT; Problem-solving therapy; Self-harm; Self-injury; Self-poisoning.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures
Similar articles
-
Psychosocial interventions for self-harm in adults.Cochrane Database Syst Rev. 2021 Apr 22;4(4):CD013668. doi: 10.1002/14651858.CD013668.pub2. Cochrane Database Syst Rev. 2021. PMID: 33884617 Free PMC article.
-
MIDSHIPS: multicentre intervention designed for self-harm using interpersonal problem-solving: protocol for a randomised controlled feasibility study.Trials. 2014 May 10;15:163. doi: 10.1186/1745-6215-15-163. Trials. 2014. PMID: 24886683 Free PMC article. Clinical Trial.
-
Psychoeducation with problem-solving (PEPS) therapy for adults with personality disorder: a pragmatic randomised controlled trial to determine the clinical effectiveness and cost-effectiveness of a manualised intervention to improve social functioning.Health Technol Assess. 2016 Jul;20(52):1-250. doi: 10.3310/hta20520. Health Technol Assess. 2016. PMID: 27431341 Free PMC article. Clinical Trial.
-
A pragmatic randomised controlled trial and economic evaluation of family therapy versus treatment as usual for young people seen after second or subsequent episodes of self-harm: the Self-Harm Intervention - Family Therapy (SHIFT) trial.Health Technol Assess. 2018 Mar;22(12):1-222. doi: 10.3310/hta22120. Health Technol Assess. 2018. PMID: 29532784 Free PMC article. Clinical Trial.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
-
Psychosocial interventions for self-harm in adults.Cochrane Database Syst Rev. 2021 Apr 22;4(4):CD013668. doi: 10.1002/14651858.CD013668.pub2. Cochrane Database Syst Rev. 2021. PMID: 33884617 Free PMC article.
-
Cost-effectiveness of psychological and psychosocial interventions for adults, children and young people who have self-harmed.BMJ Ment Health. 2024 Nov 5;27(1):e301220. doi: 10.1136/bmjment-2024-301220. BMJ Ment Health. 2024. PMID: 39500599 Free PMC article.
-
Evaluation of the impact and implementation of a national clinical programme for the management of self-harm in hospital emergency departments: study protocol for a natural experiment.BMJ Open. 2021 Dec 24;11(12):e055962. doi: 10.1136/bmjopen-2021-055962. BMJ Open. 2021. PMID: 34952886 Free PMC article.
-
Understanding suicidal pathways through the lens of a Dual-System Model of Suicidality in real-time: The potential of ecological momentary assessments.Front Psychiatry. 2022 Nov 28;13:899500. doi: 10.3389/fpsyt.2022.899500. eCollection 2022. Front Psychiatry. 2022. PMID: 36518367 Free PMC article. Review.
References
-
- Griffin E, McTernan N, Wrigley C, Nicholson S, Arensman E, Williamson E, Corcoran P. National Self-Harm Registry Ireland Annual Report 2018. National Suicide Research Foundation: Cork; 2019.
Grants and funding
LinkOut - more resources
Full Text Sources