The Role of Stress, Trauma, and Negative Affect in Alcohol Misuse and Alcohol Use Disorder in Women
- PMID: 32832310
- PMCID: PMC7431322
- DOI: 10.35946/arcr.v40.2.05
The Role of Stress, Trauma, and Negative Affect in Alcohol Misuse and Alcohol Use Disorder in Women
Abstract
Recent evidence indicates that the United States is facing a public health crisis of alcohol misuse and alcohol use disorder (AUD), which has been fueled in part by dramatic rises in binge and heavy drinking and prevalence of AUD in women. Historically, alcohol misuse and AUD have been more prevalent in men than in women. However, recent evidence on data from the past decade shows increases in AUD prevalence rates that are associated with substantially higher binge and heavy drinking and AUD prevalence in women compared to men. This paper first addresses the key roles of stress, trauma, childhood maltreatment, negative affect, and mood and anxiety disorders; sex differences in the presentation of these psychosocial and psychological factors; and their contributions to alcohol misuse, escalation to binge and heavy drinking, and transition to AUD in women. Also examined are potential central and peripheral biological mechanisms by which stressors and traumatic experiences, as well as chronic stress states-including depression and anxiety-may facilitate differential pathways to alcohol misuse, escalation, and transition to AUD in women. Finally, this paper discusses major gaps in the literature on sex differences in these areas as well as the need for greater research on sex-specific pathways to alcohol misuse and transition to AUD, so as to support a more comprehensive understanding of AUD etiology and for the development of new strategies for prevention and treatment of alcohol misuse and AUD in women.
Keywords: alcohol craving; child maltreatment; early trauma; girls and women; sex differences.
Conflict of interest statement
Financial Disclosure The authors declare that they have no competing financial interests.
Figures


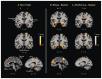
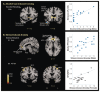
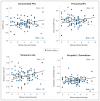
References
-
- Vos T, Abajobir AA, Abbafati C, et al. for GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. - DOI - PMC - PubMed
-
- Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74(9):911–923. doi: 10.1001/jamapsychiatry.2017.2161. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

