Prevention of HBV Recurrence after Liver Transplant: A Review
- PMID: 32832395
- PMCID: PMC7438351
- DOI: 10.14218/JCTH.2020.00003
Prevention of HBV Recurrence after Liver Transplant: A Review
Abstract
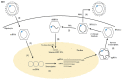
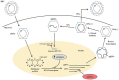
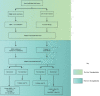
Globally, hepatitis B virus (HBV) infection is recognized as a major risk factor for the development of hepatocellular carcinoma, and HBV-induced liver failure is one of the leading indications for liver transplantation. Until about two decades ago, liver transplantation in patients with chronic HBV infection was a relative contraindication, due to high risk of viral replication with the use of immunosuppressants which could result in graft infection. In the 1990s, hepatitis B immunoglobulin (HBIg) use significantly reduced the risk of graft infection, improving outcomes of liver transplant in patients with chronic HBV infection. However, very high costs, especially with the need for long-term use, became a major concern. With the advent of nucleos(t)ide analogs (NAs), there was less need for high-dose, long-term HBIg use to prevent HBV recurrence. Lamivudine was initially used but resistance soon became a major issue. This was followed by more potent NAs, such as entecavir and tenofovir, emerging as the more preferred agents. Additionally, the use of these antiviral agents (HBIg and/or NAs) have made it possible to use the grafts from donors with positivity for hepatitis B core antibody, allowing for expansion of the donor pool. Nevertheless, there is no consensus on management protocols, which vary significantly amongst centers. In this review, we appraise studies on management strategies used and the role of active vaccination in the prevention of HBV recurrence in post-liver transplant patients.
Keywords: Hepatitis B; Liver transplant; Recurrence; Vaccination.
© 2020 Authors.
Conflict of interest statement
The authors have no conflict of interests related to this publication.
Figures
Similar articles
-
Prevention of lamivudine-resistant hepatitis B recurrence after liver transplantation with entecavir plus tenofovir combination therapy and perioperative hepatitis B immunoglobulin only.Transpl Infect Dis. 2011 Jun;13(3):299-302. doi: 10.1111/j.1399-3062.2010.00591.x. Epub 2010 Dec 16. Transpl Infect Dis. 2011. PMID: 21159112
-
Nucleos(t)ide analog(s) prophylaxis after hepatitis B immunoglobulin withdrawal against hepatitis B and D recurrence after liver transplantation.Transpl Infect Dis. 2016 Oct;18(5):667-673. doi: 10.1111/tid.12575. Epub 2016 Sep 7. Transpl Infect Dis. 2016. PMID: 27421122
-
Review of the pharmacological management of hepatitis B viral infection before and after liver transplantation.World J Gastroenterol. 2013 Dec 28;19(48):9189-97. doi: 10.3748/wjg.v19.i48.9189. World J Gastroenterol. 2013. PMID: 24409047 Free PMC article. Review.
-
Prophylaxis against hepatitis B virus recurrence after liver transplantation: a registry study.World J Gastroenterol. 2015 Jan 14;21(2):584-92. doi: 10.3748/wjg.v21.i2.584. World J Gastroenterol. 2015. PMID: 25593480 Free PMC article.
-
Antiviral prophylaxis against hepatitis B recurrence after liver transplantation: Current concepts.Liver Int. 2021 Jul;41(7):1448-1461. doi: 10.1111/liv.14860. Epub 2021 Mar 12. Liver Int. 2021. PMID: 33656809 Review.
Cited by
-
Global Perspectives on the Hepatitis B Vaccination: Challenges, Achievements, and the Road to Elimination by 2030.Vaccines (Basel). 2024 Mar 9;12(3):288. doi: 10.3390/vaccines12030288. Vaccines (Basel). 2024. PMID: 38543922 Free PMC article. Review.
-
Liver transplantation for HBV-related liver disease: Impact of prophylaxis for HBV on HCC recurrence.JHEP Rep. 2024 Nov 23;7(3):101278. doi: 10.1016/j.jhepr.2024.101278. eCollection 2025 Mar. JHEP Rep. 2024. PMID: 40041120 Free PMC article.
-
Role of lower dose hepatitis B immune globulin prophylaxis in liver transplantation: A single center perspective.Hepatol Forum. 2023 Jan 17;4(1):3-6. doi: 10.14744/hf.2022.2022.0030. eCollection 2023 Jan. Hepatol Forum. 2023. PMID: 36843892 Free PMC article.
-
Long-term outcomes of liver transplantation using grafts from donors with active hepatitis B virus replication: a multicenter cohort study.Ann Surg Treat Res. 2023 Apr;104(4):183-194. doi: 10.4174/astr.2023.104.4.183. Epub 2023 Mar 31. Ann Surg Treat Res. 2023. PMID: 37051154 Free PMC article.
-
Early intrahepatic recurrence of HBV infection in liver transplant recipients despite antiviral prophylaxis.JHEP Rep. 2023 Mar 10;5(6):100728. doi: 10.1016/j.jhepr.2023.100728. eCollection 2023 Jun. JHEP Rep. 2023. PMID: 37122357 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
