Herb-induced Liver Injury in Asia and Current Role of RUCAM for Causality Assessment in 11,160 Published Cases
- PMID: 32832401
- PMCID: PMC7438347
- DOI: 10.14218/JCTH.2020.00009
Herb-induced Liver Injury in Asia and Current Role of RUCAM for Causality Assessment in 11,160 Published Cases
Abstract
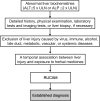
Herb-induced liver injuries (HILI) by traditional herbal medicines are particular challenges in Asian countries, with issues over the best approach to establish causality. The aim of the current analysis was to provide an overview on how causality was assessed in HILI cases from Asian countries and whether the Roussel Uclaf Causality Assessment Method (RUCAM) was the preferred diagnostic algorithm, as shown before in worldwide evaluated cases of drug-induced liver injury (DILI). Using the PubMed database, publications in English language were preferred to allow for reevaluation by peers. Overall 11,160 HILI cases have assessed causality using RUCAM and were published by first authors working in Asian countries. With 21 evaluable reports, most publications came from mainland China, with Hong Kong and Taiwan, followed by Korea (n=15), Singapore (n=2), and Japan (n=1), while other Asian countries were not contributory. Most publications provided case and RUCAM data of good quality. For better presentation of future cases, however, the following recommendations are given: (1) preference of prospective study design with use of the updated RUCAM version; (2) clear separation of HILI cohorts from those of other herbal products or DILI; (3) case series for epidemiology studies should contain many essential data, possibly also as supplementary material; (4) otherwise, preference of single case reports providing individual case data and RUCAM-based causality gradings, and applying liver test threshold values; and (5) publication in English language journals. In conclusion, China and Korea are top in presenting RUCAM-based HILI cases, other Asian countries are encouraged to follow.
Keywords: Drug induced liver injury; Herb induced liver injury; Liver injury; RUCAM.
© 2020 Authors.
Conflict of interest statement
The authors have no conflict of interests related to this publication.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
