Profound Functional Suppression of Tumor-Infiltrating T-Cells in Ovarian Cancer Patients Can Be Reversed Using PD-1-Blocking Antibodies or DARPin® Proteins
- PMID: 32832572
- PMCID: PMC7424497
- DOI: 10.1155/2020/7375947
Profound Functional Suppression of Tumor-Infiltrating T-Cells in Ovarian Cancer Patients Can Be Reversed Using PD-1-Blocking Antibodies or DARPin® Proteins
Abstract
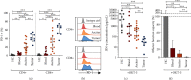
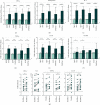
PD-1/PD-L1 blockade has revolutionized the field of immunooncology. Despite the relative success, the response rate to anti-PD-1 therapy requires further improvements. Our aim was to explore the enhancement of T-cell function by using novel PD-1-blocking proteins and compare with clinically approved monoclonal antibodies (mAbs). We isolated T-cells from the ascites and tumor of 17 patients with advanced epithelial ovarian cancer (EOC) and analyzed the effects using the mAbs nivolumab and pembrolizumab and two novel engineered ankyrin repeat proteins (DARPin® proteins). PD-1 blockade with either mAb or DARPin® molecule significantly increased the release of IFN-γ, granzyme B, IL-2, and TNF-α, demonstrating successful reinvigoration. The monovalent DARPin® protein was less effective compared to its bivalent equivalent, demonstrating that bivalency brings an additional benefit to PD-1 blockade. Overall, we found a higher fold increase of lymphokine secretion in response to the PD-1 blockade by tumor-derived T-cells; however, the absolute amounts were significantly lower compared to the release from ascites-derived T-cells. Our results demonstrate that PD-1 blockade can only partially reinvigorate functionally suppressed T-cells from EOC patients. This warrants further investigation preferably in combination with other therapeutics. The study provides an early pilot proof-of-concept for the potential use of DARPin® proteins as eligible alternative scaffold proteins to block PD-1.
Copyright © 2020 Emelie Foord et al.
Conflict of interest statement
JK, MM, CH, DSc, DV, UF, DSn, and VL are employed by Molecular Partners AG and hold options or shares in the company. BK was employed by Molecular Partners AG and holds options or shares in the company. BK is now an employee and shareholder of AstraZeneca. No potential conflicts of interest were disclosed by the other authors.
Figures


Similar articles
-
Inhibition of T-cell-mediated immune response via the PD-1/ PD-L1 axis in cholangiocarcinoma cells.Eur J Pharmacol. 2021 Apr 15;897:173960. doi: 10.1016/j.ejphar.2021.173960. Epub 2021 Feb 19. Eur J Pharmacol. 2021. PMID: 33617828
-
The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies.Nat Immunol. 2020 Nov;21(11):1346-1358. doi: 10.1038/s41590-020-0769-3. Epub 2020 Aug 31. Nat Immunol. 2020. PMID: 32868929
-
Blocking Wnt/β-catenin Signal Amplifies Anti-PD-1 Therapeutic Efficacy by Inhibiting Tumor Growth, Migration, and Promoting Immune Infiltration in Glioblastomas.Mol Cancer Ther. 2021 Jul;20(7):1305-1315. doi: 10.1158/1535-7163.MCT-20-0825. Epub 2021 May 17. Mol Cancer Ther. 2021. PMID: 34001635
-
Immunotherapy for the treatment of breast cancer: checkpoint blockade, cancer vaccines, and future directions in combination immunotherapy.Clin Adv Hematol Oncol. 2016 Nov;14(11):922-933. Clin Adv Hematol Oncol. 2016. PMID: 27930644 Review.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
Cited by
-
NSCLC: from tumorigenesis, immune checkpoint misuse to current and future targeted therapy.Front Immunol. 2024 Feb 7;15:1342086. doi: 10.3389/fimmu.2024.1342086. eCollection 2024. Front Immunol. 2024. PMID: 38384472 Free PMC article. Review.
-
Revolutionization in Cancer Therapeutics via Targeting Major Immune Checkpoints PD-1, PD-L1 and CTLA-4.Pharmaceuticals (Basel). 2022 Mar 9;15(3):335. doi: 10.3390/ph15030335. Pharmaceuticals (Basel). 2022. PMID: 35337133 Free PMC article. Review.
-
The crosstalk between immune cells and tumor pyroptosis: advancing cancer immunotherapy strategies.J Exp Clin Cancer Res. 2024 Jul 10;43(1):190. doi: 10.1186/s13046-024-03115-7. J Exp Clin Cancer Res. 2024. PMID: 38987821 Free PMC article. Review.
-
Directed Evolution of PD-L1-Targeted Affibodies by mRNA Display.ACS Chem Biol. 2022 Jun 17;17(6):1543-1555. doi: 10.1021/acschembio.2c00218. Epub 2022 May 25. ACS Chem Biol. 2022. PMID: 35611948 Free PMC article.
-
The role of interferons in ovarian cancer progression: Hinderer or promoter?Front Immunol. 2022 Dec 21;13:1087620. doi: 10.3389/fimmu.2022.1087620. eCollection 2022. Front Immunol. 2022. PMID: 36618371 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

