SirT7 auto-ADP-ribosylation regulates glucose starvation response through mH2A1
- PMID: 32832656
- PMCID: PMC7439345
- DOI: 10.1126/sciadv.aaz2590
SirT7 auto-ADP-ribosylation regulates glucose starvation response through mH2A1
Abstract
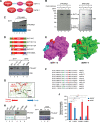
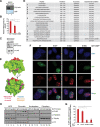
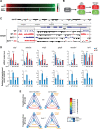
Sirtuins are key players of metabolic stress response. Originally described as deacetylases, some sirtuins also exhibit poorly understood mono-adenosine 5'-diphosphate (ADP)-ribosyltransferase (mADPRT) activity. We report that the deacetylase SirT7 is a dual sirtuin, as it also features auto-mADPRT activity. SirT7 mADPRT occurs at a previously undefined active site, and its abrogation alters SirT7 chromatin distribution. We identify an epigenetic pathway by which ADP-ribosyl-SirT7 is recognized by the ADP-ribose reader mH2A1.1 under glucose starvation, inducing SirT7 relocalization to intergenic regions. SirT7 promotes mH2A1 enrichment in a subset of nearby genes, many of them involved in second messenger signaling, resulting in their specific up- or down-regulation. The expression profile of these genes under calorie restriction is consistently abrogated in SirT7-deficient mice, resulting in impaired activation of autophagy. Our work provides a novel perspective on sirtuin duality and suggests a role for SirT7/mH2A1.1 axis in glucose homeostasis and aging.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
-
- Bosch-Presegué L., Vaquero A., Sirtuins in stress response: Guardians of the genome. Oncogene 33, 3764–3775 (2014). - PubMed
-
- Caban C. E., Ginsburg A., Glutamine synthetase adenylyltransferase from Escherichia coli: Purification and physical and chemical properties. Biochemistry 15, 1569–1580 (1976). - PubMed
-
- Nureki O., Vassylyev D. G., Tateno M., Shimada A., Nakama T., Fukai S., Konno M., Hendrickson T. L., Schimmel P., Yokoyama S., Enzyme structure with two catalytic sites for double-sieve selection of substrate. Science 280, 578–582 (1998). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

