Shattering barriers toward clinically meaningful MSC therapies
- PMID: 32832666
- PMCID: PMC7439491
- DOI: 10.1126/sciadv.aba6884
Shattering barriers toward clinically meaningful MSC therapies
Abstract
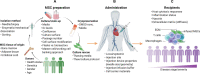
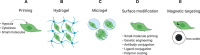
More than 1050 clinical trials are registered at FDA.gov that explore multipotent mesenchymal stromal cells (MSCs) for nearly every clinical application imaginable, including neurodegenerative and cardiac disorders, perianal fistulas, graft-versus-host disease, COVID-19, and cancer. Several companies have or are in the process of commercializing MSC-based therapies. However, most of the clinical-stage MSC therapies have been unable to meet primary efficacy end points. The innate therapeutic functions of MSCs administered to humans are not as robust as demonstrated in preclinical studies, and in general, the translation of cell-based therapy is impaired by a myriad of steps that introduce heterogeneity. In this review, we discuss the major clinical challenges with MSC therapies, the details of these challenges, and the potential bioengineering approaches that leverage the unique biology of MSCs to overcome the challenges and achieve more potent and versatile therapies.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Friedenstein A. J., Gorskaja J. F., Kulagina N. N., Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 4, 267–274 (1976). - PubMed
-
- A study of CYP-001 for the treatment of steroid-resistant acute graft versus host disease; https://clinicaltrials.gov/ct2/show/NCT02923375.
-
- Prochymal® (human adult stem cells) intravenous infusion following acute myocardial infarction (AMI); https://clinicaltrials.gov/ct2/show/NCT00877903.
-
- Safety and efficacy of intravenous autologous mesenchymal stem cells for MS: A phase 2 proof of concept study (MESCAMS); https://clinicaltrials.gov/ct2/show/NCT02239393.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

