IL-10-producing Tfh cells accumulate with age and link inflammation with age-related immune suppression
- PMID: 32832688
- PMCID: PMC7439492
- DOI: 10.1126/sciadv.abb0806
IL-10-producing Tfh cells accumulate with age and link inflammation with age-related immune suppression
Abstract
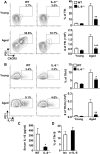
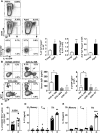
Aging results in profound immune dysfunction, resulting in the decline of vaccine responsiveness previously attributed to irreversible defects in the immune system. In addition to increased interleukin-6 (IL-6), we found aged mice exhibit increased systemic IL-10 that requires forkhead box P3-negative (FoxP3-), but not FoxP3+, CD4+T cells. Most IL-10-producing cells manifested a T follicular helper (Tfh) phenotype and required the Tfh cytokines IL-6 and IL-21 for their accrual, so we refer to them as Tfh10 cells. IL-21 was also required to maintain normal serum levels of IL-6 and IL-10. Notably, antigen-specific Tfh10 cells arose after immunization of aged mice, and neutralization of IL-10 receptor signaling significantly restored Tfh-dependent antibody responses, whereas depletion of FoxP3+ regulatory and follicular regulatory cells did not. Thus, these data demonstrate that immune suppression with age is reversible and implicate Tfh10 cells as an intriguing link between "inflammaging" and impaired immune responses with age.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures




References
-
- Lio D., Scola L., Crivello A., Colonna-Romano G., Candore G., Bonafè M., Cavallone L., Franceschi C., Caruso C., Gender-specific association between -1082 IL-10 promoter polymorphism and longevity. Genes Immun. 3, 30–33 (2002). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

