Postprandial Hyperglycemia Lowering Effect of the Isolated Compounds from Olive Mill Wastes - An Inhibitory Activity and Kinetics Studies on α-Glucosidase and α-Amylase Enzymes
- PMID: 32832761
- PMCID: PMC7439263
- DOI: 10.1021/acsomega.0c01622
Postprandial Hyperglycemia Lowering Effect of the Isolated Compounds from Olive Mill Wastes - An Inhibitory Activity and Kinetics Studies on α-Glucosidase and α-Amylase Enzymes
Abstract
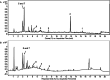
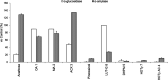
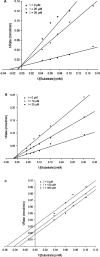
In the present study, we isolated seven compounds from olive mill wastes (OMW), one of them being novel, and investigated their antidiabetic potential through inhibition of α-glucosidase and α-amylase enzymes. To assist the possible characterization of the mechanisms involved, we analyzed the inhibitory kinetics of the active compounds. Oleanolic acid 1, maslinic acid 2, 1-acetoxypinoresinol 3, and luteolin-7-O-β-d-glucoside 6 exhibited stronger inhibitory activity against both enzymes, with IC50 values less than or close to that of acarbose. Other compounds pinoresinol and hydroxytyrosol-containing compounds (hydroxytyrosol acetate 4, hydroxytyrosol 7, and the novel one, 3,4-dihydroxyphenyl-2-methoxyethanol 5) showed weak activity against both enzymes (IC50 > 500 μM). Our findings show that, first, the esterification of C-1 of the furofuran ring is the key feature for the stronger activity of 1-acetoxypinoresinol 3 against both enzymes (IC50 = 13.9 and 313 μM for α-amylase and α-glucosidase, respectively), as compared to pinoresinol; second, the oleanane skeletons of the triterpenes (1 and 2) are optimum for the α-glucosidase and α-amylase inhibitory activities, while the hydroxytyrosol moiety may be responsible for the weak activities of 4, 5, and 7. Additionally, kinetics analysis of 1, 6, and 3 revealed that they inhibit α-glucosidase in mixed-type, noncompetitive, and uncompetitive mechanisms, respectively. We confirmed their mechanisms by measuring their affinity for the enzyme (K i), and they all (1, 6, and 3) had a higher affinity for the enzyme, K i > 1. This work adds more value to OMW for further studies as a potential source of lead antidiabetic compounds for the prevention and/or treatment of type 2 diabetes.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
The α-Glucosidase and α-Amylase Enzyme Inhibitory of Hydroxytyrosol and Oleuropein.J Oleo Sci. 2015;64(8):835-43. doi: 10.5650/jos.ess15026. J Oleo Sci. 2015. PMID: 26235001
-
Pentacyclic triterpenes as α-glucosidase and α-amylase inhibitors: Structure-activity relationships and the synergism with acarbose.Bioorg Med Chem Lett. 2017 Nov 15;27(22):5065-5070. doi: 10.1016/j.bmcl.2017.09.027. Epub 2017 Sep 14. Bioorg Med Chem Lett. 2017. PMID: 28964635
-
Flavonoids and its derivatives from Callistephus chinensis flowers and their inhibitory activities against alpha-glucosidase.EXCLI J. 2013 Nov 19;12:956-66. eCollection 2013. EXCLI J. 2013. PMID: 27298611 Free PMC article.
-
Flavonoids as dual-target inhibitors against α-glucosidase and α-amylase: a systematic review of in vitro studies.Nat Prod Bioprospect. 2024 Jan 8;14(1):4. doi: 10.1007/s13659-023-00424-w. Nat Prod Bioprospect. 2024. PMID: 38185713 Free PMC article. Review.
-
The α-Amylase and α-Glucosidase Inhibition Capacity of Grape Pomace: A Review.Food Bioproc Tech. 2023;16(4):691-703. doi: 10.1007/s11947-022-02895-0. Epub 2022 Aug 30. Food Bioproc Tech. 2023. PMID: 36062030 Free PMC article. Review.
Cited by
-
Antimicrobial and Wound Healing Properties of FeO Fabricated Chitosan/PVA Nanocomposite Sponge.Antibiotics (Basel). 2021 May 3;10(5):524. doi: 10.3390/antibiotics10050524. Antibiotics (Basel). 2021. PMID: 34063621 Free PMC article.
-
Recent Advances Regarding the Molecular Mechanisms of Triterpenic Acids: A Review (Part II).Int J Mol Sci. 2022 Aug 10;23(16):8896. doi: 10.3390/ijms23168896. Int J Mol Sci. 2022. PMID: 36012159 Free PMC article. Review.
-
HPLC-DAD-MS Characterization, Antioxidant Activity, α-amylase Inhibition, Molecular Docking, and ADMET of Flavonoids from Fenugreek Seeds.Molecules. 2023 Nov 27;28(23):7798. doi: 10.3390/molecules28237798. Molecules. 2023. PMID: 38067527 Free PMC article.
-
Impact of the Food Matrix on the Antioxidant and Hypoglycemic Effects of Betalains from Red Prickly Pear Juice After In Vitro Digestion.Foods. 2025 May 15;14(10):1757. doi: 10.3390/foods14101757. Foods. 2025. PMID: 40428536 Free PMC article.
-
Multifaceted biomedical applications of biogenic titanium dioxide nanoparticles fabricated by marine actinobacterium Streptomyces vinaceusdrappus AMG31.Sci Rep. 2025 Jun 23;15(1):20244. doi: 10.1038/s41598-025-00541-1. Sci Rep. 2025. PMID: 40550842 Free PMC article.
References
-
- Gin H.; Rigalleau V. Post-Prandial Hyperglycemia. Post-Prandial Hyperglycemia and Diabetes. Diabetes Metab. 2000, 26, 265–272. - PubMed
-
- Eringa E. C.; Serne E. H.; Meijer R. I.; Schalkwijk C. G.; Houben A. J. H. M.; Stehouwer C. D. A.; Smulders Y. M.; Van Hinsbergh V. W. M. Endothelial Dysfunction in (Pre) Diabetes: Characteristics, Causative Mechanisms and Pathogenic Role in Type 2 Diabetes. Rev. Endocr. Metab. Disord. 2013, 14, 39–48. 10.1007/s11154-013-9239-7. - DOI - PubMed
-
- Taslimi P.; Aslan H. E.; Demir Y.; Oztaskin N.; Maraş A.; Gulçin İ.; Beydemir S.; Goksu S. Diarylmethanon, Bromophenol and Diarylmethane Compounds: Discovery of Potent Aldose Reductase, α-Amylase and α-Glycosidase Inhibitors as New Therapeutic Approach in Diabetes and Functional Hyperglycemia. Int. J. Biol. Macromol. 2018, 119, 857–863. 10.1016/j.ijbiomac.2018.08.004. - DOI - PubMed
LinkOut - more resources
Full Text Sources

