Development of Cuboidal KNbO3@α-Fe2O3 Hybrid Nanostructures for Improved Photocatalytic and Photoelectrocatalytic Applications
- PMID: 32832802
- PMCID: PMC7439387
- DOI: 10.1021/acsomega.0c02646
Development of Cuboidal KNbO3@α-Fe2O3 Hybrid Nanostructures for Improved Photocatalytic and Photoelectrocatalytic Applications
Abstract
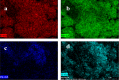
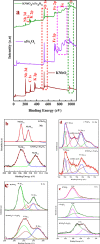
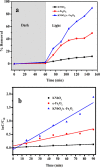
Monophasic and hybrid nanostructures of KNbO3 and α-Fe2O3 have been prepared using a hydrothermal process for photoelectrocatalytic and photocatalytic applications. Powder X-ray diffraction studies showed the formation of KNbO3, α-Fe2O3, and KNbO3/α-Fe2O3 with average grain sizes of 18.3, 11.5, and 26.1 nm and Brunauer-Emmett-Teller (BET) specific surface areas of 4, 100, and 20 m2/gm, respectively. Under simulated solar irradiation, the as-prepared heterostructure shows enhanced photoelectrocatalytic oxygen evolution reaction (OER) activity compared to pristine KNbO3 and α-Fe2O3. Significant photocatalytic activity of as-synthesized KNbO3/α-Fe2O3 heterostructure photocatalyst was obtained for removal of methylene blue organic dye under visible light, and the percentage activity was found to be 11, 49, and 89% for KNbO3, α-Fe2O3, and KNbO3/α-Fe2O3 photocatalysts, respectively. The dielectric constant was found to be 250.2, 65.2, and 251.5 for KNbO3, α-Fe2O3, and KNbO3/α-Fe2O3 heterostructure, respectively, at 50 °C and 500 kHz frequency.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








Similar articles
-
Preparation of CdS and Bi2S3 quantum dots co-decorated perovskite-type KNbO3 ternary heterostructure with improved visible light photocatalytic activity and stability for phenol degradation.Dalton Trans. 2018 Oct 30;47(42):15232-15245. doi: 10.1039/c8dt03094d. Dalton Trans. 2018. PMID: 30320859
-
Synthesis and Characterization of an α-Fe2O3-Decorated g-C3N4 Heterostructure for the Photocatalytic Removal of MO.Molecules. 2022 Feb 21;27(4):1442. doi: 10.3390/molecules27041442. Molecules. 2022. PMID: 35209230 Free PMC article.
-
Synthesis and characterization of an α-Fe2O3/ZnTe heterostructure for photocatalytic degradation of Congo red, methyl orange and methylene blue.RSC Adv. 2020 Dec 21;10(73):44997-45007. doi: 10.1039/d0ra06866g. eCollection 2020 Dec 17. RSC Adv. 2020. PMID: 35516253 Free PMC article.
-
A strategy to enhance the photocatalytic efficiency of α-Fe2O3.Chemosphere. 2021 May;270:129498. doi: 10.1016/j.chemosphere.2020.129498. Epub 2021 Jan 2. Chemosphere. 2021. PMID: 33422995
-
A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants.J Environ Manage. 2020 Mar 15;258:110050. doi: 10.1016/j.jenvman.2019.110050. Epub 2020 Jan 7. J Environ Manage. 2020. PMID: 31929077 Review.
Cited by
-
Optimizations of Liquid Phase Deposition Processes for Enhanced Photoelectrocatalytic Activities of Tungsten Oxide Thin Films.ACS Omega. 2024 Sep 4;9(37):38788-38797. doi: 10.1021/acsomega.4c04738. eCollection 2024 Sep 17. ACS Omega. 2024. PMID: 39310131 Free PMC article.
-
Enhanced Visible-Light Absorption of Fe2O3 Covered by Activated Carbon for Multifunctional Purposes: Tuning the Structural, Electronic, Optical, and Magnetic Properties.ACS Omega. 2021 Oct 18;6(42):28334-28346. doi: 10.1021/acsomega.1c04526. eCollection 2021 Oct 26. ACS Omega. 2021. PMID: 34723030 Free PMC article.
-
Antioxidant and Anti-inflammatory Applications of Aerva persica Aqueous-Root Extract-Mediated Synthesis of ZnO Nanoparticles.ACS Omega. 2024 Mar 29;9(14):15882-15892. doi: 10.1021/acsomega.3c08143. eCollection 2024 Apr 9. ACS Omega. 2024. PMID: 38617686 Free PMC article.
-
Nonenzymatic Electrochemical Sensing of Glucose Using Hydrothermally Synthesized High-Specific-Surface-Area SnO2 Nanoparticles.ACS Omega. 2025 Jul 10;10(28):30639-30650. doi: 10.1021/acsomega.5c02597. eCollection 2025 Jul 22. ACS Omega. 2025. PMID: 40727770 Free PMC article.
-
Progress in Promising Semiconductor Materials for Efficient Photoelectrocatalytic Hydrogen Production.Molecules. 2024 Jan 5;29(2):289. doi: 10.3390/molecules29020289. Molecules. 2024. PMID: 38257202 Free PMC article. Review.
References
-
- Dufour J.; Serrano D. P.; Gálvez J. L.; Moreno J.; González A. Hydrogen production from fossil fuels: Life cycle assessment of technologies with low greenhouse gas emissions. Energy Fuels 2011, 25, 2194–2202. 10.1021/ef200124d. - DOI
-
- Zhao Y.; Hoivik N.; Wang K. Recent advance on engineering titanium dioxide nanotubes for photochemical and photoelectrochemical water splitting. Nano Energy 2016, 30, 728–744. 10.1016/j.nanoen.2016.09.027. - DOI
-
- Kumar S.; Malik T.; Sharma D.; Ganguli A. K. NaNbO3/MoS2 and NaNbO3/BiVO4 core/shell nanostructures for photoelectrochemical hydrogen generation. ACS Appl. Nano Mater. 2019, 2, 2651–2662. 10.1021/acsanm.9b00098. - DOI
-
- Xue X.; Zang W.; Deng P.; Wang Q.; Xing L. Piezo-potential enhanced photocatalytic degradation of organic dye using ZnO nanowires. Nano Energy 2015, 13, 414–422. 10.1016/j.nanoen.2015.02.029. - DOI
LinkOut - more resources
Full Text Sources

