A CRISPR-associated factor Csa3a regulates DNA damage repair in Crenarchaeon Sulfolobus islandicus
- PMID: 32833023
- PMCID: PMC7515695
- DOI: 10.1093/nar/gkaa694
A CRISPR-associated factor Csa3a regulates DNA damage repair in Crenarchaeon Sulfolobus islandicus
Abstract
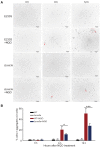
CRISPR-Cas system provides acquired immunity against invasive genetic elements in prokaryotes. In both bacteria and archaea, transcriptional factors play important roles in regulation of CRISPR adaptation and interference. In the model Crenarchaeon Sulfolobus islandicus, a CRISPR-associated factor Csa3a triggers CRISPR adaptation and activates CRISPR RNA transcription for the immunity. However, regulation of DNA repair systems for repairing the genomic DNA damages caused by the CRISPR self-immunity is less understood. Here, according to the transcriptome and reporter gene data, we found that deletion of the csa3a gene down-regulated the DNA damage response (DDR) genes, including the ups and ced genes. Furthermore, in vitro analyses demonstrated that Csa3a specifically bound the DDR gene promoters. Microscopic analysis showed that deletion of csa3a significantly inhibited DNA damage-induced cell aggregation. Moreover, the flow cytometry study and survival rate analysis revealed that the csa3a deletion strain was more sensitive to the DNA-damaging reagent. Importantly, CRISPR self-targeting and DNA transfer experiments revealed that Csa3a was involved in regulating Ups- and Ced-mediated repair of CRISPR-damaged host genomic DNA. These results explain the interplay between Csa3a functions in activating CRISPR adaptation and DNA repair systems, and expands our understanding of the lost link between CRISPR self-immunity and genome stability.
© The Author(s) 2020. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





Similar articles
-
Coupling transcriptional activation of CRISPR-Cas system and DNA repair genes by Csa3a in Sulfolobus islandicus.Nucleic Acids Res. 2017 Sep 6;45(15):8978-8992. doi: 10.1093/nar/gkx612. Nucleic Acids Res. 2017. PMID: 28911114 Free PMC article.
-
Genetic determinants of PAM-dependent DNA targeting and pre-crRNA processing in Sulfolobus islandicus.RNA Biol. 2013 May;10(5):738-48. doi: 10.4161/rna.23798. Epub 2013 Feb 7. RNA Biol. 2013. PMID: 23392249 Free PMC article.
-
Transcriptional regulator-mediated activation of adaptation genes triggers CRISPR de novo spacer acquisition.Nucleic Acids Res. 2015 Jan;43(2):1044-55. doi: 10.1093/nar/gku1383. Epub 2015 Jan 7. Nucleic Acids Res. 2015. PMID: 25567986 Free PMC article.
-
CRISPR-mediated defense mechanisms in the hyperthermophilic archaeal genus Sulfolobus.RNA Biol. 2013 May;10(5):671-8. doi: 10.4161/rna.24154. Epub 2013 Mar 27. RNA Biol. 2013. PMID: 23535277 Free PMC article. Review.
-
CRISPR-Cas adaptation: insights into the mechanism of action.Nat Rev Microbiol. 2016 Feb;14(2):67-76. doi: 10.1038/nrmicro.2015.14. Epub 2016 Jan 11. Nat Rev Microbiol. 2016. PMID: 26751509 Review.
Cited by
-
Cyclic Tetra-Adenylate (cA4) Recognition by Csa3; Implications for an Integrated Class 1 CRISPR-Cas Immune Response in Saccharolobus solfataricus.Biomolecules. 2021 Dec 9;11(12):1852. doi: 10.3390/biom11121852. Biomolecules. 2021. PMID: 34944496 Free PMC article.
-
Identification of novel components of the Ced and Ups systems in Saccharolobus islandicus REY15A.mLife. 2025 Feb 23;4(1):17-28. doi: 10.1002/mlf2.12163. eCollection 2025 Feb. mLife. 2025. PMID: 40026581 Free PMC article.
-
Cas1 and Fen1 Display Equivalent Functions During Archaeal DNA Repair.Front Microbiol. 2022 Apr 15;13:822304. doi: 10.3389/fmicb.2022.822304. eCollection 2022. Front Microbiol. 2022. PMID: 35495653 Free PMC article.
-
A Haloarchaeal Transcriptional Regulator That Represses the Expression of CRISPR-Associated Genes.Microorganisms. 2024 Aug 27;12(9):1772. doi: 10.3390/microorganisms12091772. Microorganisms. 2024. PMID: 39338447 Free PMC article.
References
-
- Barrangou R., Fremaux C., Deveau H., Richards M., Boyaval P., Moineau S., Romero D.A., Horvath P.. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007; 315:1709–1712. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

