Autoantibodies Blocking M3 Muscarinic Receptors Cause Postganglionic Cholinergic Dysautonomia
- PMID: 32833276
- PMCID: PMC7674213
- DOI: 10.1002/ana.25882
Autoantibodies Blocking M3 Muscarinic Receptors Cause Postganglionic Cholinergic Dysautonomia
Abstract
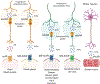
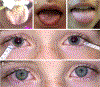
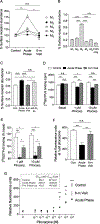
A 10-year-old girl presented with ileus, urinary retention, dry mouth, lack of tears, fixed dilated pupils, and diffuse anhidrosis 7 days after a febrile illness. We hypothesized that her syndrome was due to autoimmunity against muscarinic acetylcholine receptors, blocking their activation. Using an indirect enzyme-linked immunosorbent assay for all 5 muscarinic receptors (M1 -M5 ), we identified in the patient's serum antibodies that selectively bound to M3 receptors. In vitro functional studies confirmed that these autoantibodies selectively blocked M3 receptor activation. Thus, autoantibodies against M3 acetylcholine receptors cause acute postganglionic cholinergic dysautonomia. ANN NEUROL 2020;88:1237-1243.
© 2020 American Neurological Association.
Conflict of interest statement
POTENTIAL CONFLICTS OF INTEREST
All authors declare no conflict of interests related to this manuscript.
Figures
Similar articles
-
Inhibitory effects of muscarinic receptor autoantibodies on parasympathetic neurotransmission in Sjögren's syndrome.Arthritis Rheum. 2000 Jul;43(7):1647-54. doi: 10.1002/1529-0131(200007)43:7<1647::AID-ANR31>3.0.CO;2-P. Arthritis Rheum. 2000. PMID: 10902771
-
[The clinical significance of autoantibodies against acetylcholine muscarinic 3 receptor in primary Sjögren's syndrome].Zhonghua Nei Ke Za Zhi. 2008 Jul;47(7):563-5. Zhonghua Nei Ke Za Zhi. 2008. PMID: 19035167 Chinese.
-
Neutralization of muscarinic receptor autoantibodies by intravenous immunoglobulin in Sjögren syndrome.Hum Immunol. 2005 Apr;66(4):411-6. doi: 10.1016/j.humimm.2005.01.020. Hum Immunol. 2005. PMID: 15866705
-
Anti-M3 muscarinic acetylcholine receptor antibodies in patients with Sjögren's syndrome.Mod Rheumatol. 2013 Sep;23(5):841-5. doi: 10.1007/s10165-012-0788-5. Epub 2012 Nov 8. Mod Rheumatol. 2013. PMID: 23135882 Review.
-
Functional role of M3 muscarinic acetylcholine receptor (M3R) reactive T cells and anti-M3R autoantibodies in patients with Sjögren's syndrome.Autoimmun Rev. 2010 Jul;9(9):615-7. doi: 10.1016/j.autrev.2010.05.008. Epub 2010 May 10. Autoimmun Rev. 2010. PMID: 20462524 Review.
Cited by
-
Evaluation and management of dry mouth and its complications in rheumatology practice.Expert Rev Clin Immunol. 2024 Jan-Jun;20(1):1-19. doi: 10.1080/1744666X.2023.2268283. Epub 2024 Jan 8. Expert Rev Clin Immunol. 2024. PMID: 37823475 Free PMC article. Review.
-
Muscarinic control of cardiovascular function in humans: a review of current clinical evidence.Clin Auton Res. 2024 Feb;34(1):31-44. doi: 10.1007/s10286-024-01016-5. Epub 2024 Feb 2. Clin Auton Res. 2024. PMID: 38305989 Free PMC article. Review.
-
Gastrointestinal motility disorders in neurologic disease.J Clin Invest. 2021 Feb 15;131(4):e143771. doi: 10.1172/JCI143771. J Clin Invest. 2021. PMID: 33586685 Free PMC article. Review.
-
Covid-19-Induced Dysautonomia: A Menace of Sympathetic Storm.ASN Neuro. 2021 Jan-Dec;13:17590914211057635. doi: 10.1177/17590914211057635. ASN Neuro. 2021. PMID: 34755562 Free PMC article.
-
Reflections on the past three decades of autonomic neurology.Clin Auton Res. 2021 Feb;31(1):7-9. doi: 10.1007/s10286-020-00750-w. Epub 2021 Jan 1. Clin Auton Res. 2021. PMID: 33386431 No abstract available.
References
-
- Thomashefsky AJ, Horwitz SJ, Feingold MH. Acute autonomic neuropathy. Neurology. 1972. March;22(3):251–5. - PubMed
-
- Andersen O, Lindberg J, Modigh K, Reske-Nielsen E. Subacute dysautonomia with incomplete recovery. Acta Neurol Scand. 1972;48(4):510–9. - PubMed
-
- Harik SI, Ghandour MH, Farah FS, Afifi AK. Postganglionic cholinergic dysautonomia. Ann Neurol. 1977. April;1(4):393–6. - PubMed
-
- Inamdar S, Easton LB, Lester G. Acquired postganglionic cholinergic dysautonomia: case report and review of the literature. Pediatrics. 1982. December;70(6):976–8. - PubMed
-
- Vassallo M, Camilleri M, Caron BL, Low PA. Gastrointestinal motor dysfunction in acquired selective cholinergic dysautonomia associated with infectious mononucleosis. Gastroenterology. 1991. January;100(1):252–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

