Pd-Catalyzed Directed Thiocyanation Reaction by C-H Bond Activation
- PMID: 32833317
- PMCID: PMC7756308
- DOI: 10.1002/chem.202003521
Pd-Catalyzed Directed Thiocyanation Reaction by C-H Bond Activation
Abstract
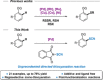
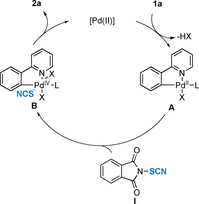
The Pd-catalyzed directed thiocyanation reaction of arenes and heteroarenes by C-H bond activation was achieved. In the presence of an electrophilic SCN source, this original methodology offered an efficient tool to access a panel of functionalized thiocyanated compounds (21 examples, up to 78 % yield). Post-functionalization reactions further demonstrated the synthetic utility of the approach by converting the SCN-containing molecules into value-added scaffolds.
Keywords: C−H activation; homogeneous catalysis; palladium; synthetic methodology; thiocyanation.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- For selected reviews, see:
-
- Jazzar R., Hitce J., Renaudat A., Sofack-Kreutzer J., Baudoin O., Chem. Eur. J. 2010, 16, 2654–2672; - PubMed
-
- Chen Z., Wang B., Zhang J., Yu W., Liu Z., Zhang Y., Org. Chem. Front. 2015, 2, 1107–1295;
Grants and funding
LinkOut - more resources
Full Text Sources

