Efficacy and safety of immune checkpoint inhibitor consolidation after chemoradiation in patients of Asian ethnicity with unresectable stage III non-small cell lung cancer: Chinese multicenter report and literature review
- PMID: 32833338
- PMCID: PMC7529561
- DOI: 10.1111/1759-7714.13631
Efficacy and safety of immune checkpoint inhibitor consolidation after chemoradiation in patients of Asian ethnicity with unresectable stage III non-small cell lung cancer: Chinese multicenter report and literature review
Abstract
Background: The PACIFIC study has defined a new standard of care for patients with unresectable stage III non-small cell lung cancer (NSCLC) in the form of immune checkpoint inhibitor (ICI) consolidation therapy. However, there is little specific data pertaining to the safety and efficacy of this approach in Chinese NSCLC patients.
Methods: This was a prospective multicenter cohort study. Between September 2018 and January 2020, patients with unresectable stage III NSCLC that had undergone chemoradiation therapy (CRT) and ICI consolidation treatment were enrolled in this study. The short-term safety, tolerability, and efficacy of ICI combination with CRT were evaluated in these patients.
Results: Of the 20 Chinese patients eligible for inclusion, 17 (85.0%) underwent concurrent CRT treatment. In these patients, a median period of 40.5 days (range: 1-85 days) passed between the end of CRT and initiation of consolidation therapy. Pneumonitis occurred in 80.0% of patients, with seven (35.0%) being diagnosed with grade 1 pneumonitis and nine (45.0%) with grade 2 pneumonitis. No patients experienced grade 3 or higher pneumonitis or other ICI-related toxicities. Lung V20 ≥ 20% was associated with higher grade 2 pneumonitis (77.8%; ≥20% vs. 18.2%; <20%, P = 0.027). The overall response rate (ORR) in these patients was 95.0%. Over a median follow-up period of 11.3 months (range: 6.2-21.8 months), 12-month PFS of these patients were 89.5% (95% CI: 76.7-100.0%), and 12 months OS was 100.0%.
Conclusions: These data indicate that ICI consolidation therapy can achieve favorable short-term efficacy, while exhibiting good safety and acceptable toxicity profiles in Chinese patients with unresectable stage III NSCLC.
Key points: Significant findings of the study This is the first report evaluating the safety and efficacy of ICI consolidation therapy after chemoradiotherapy in China. Our results indicate that ICI consolidation is associated with favorable efficacy and no severe pneumonitis incidence in Chinese patients undergoing both anti-PD-1 and anti-PD-L1 monoclonal antibody consolidation. What this study adds Post-hoc analysis of the Japanese subgroup in the PACIFIC study suggested that consolidation therapy may be associated with increased pneumonitis incidence in Asian patients. However, our findings indicate that consolidation therapy is safe and tolerable in Chinese patients with unresectable stage III NSCLC.
Keywords: Chinese; PD-1; PD-L1; pneumonitis.
© 2020 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Figures
Similar articles
-
Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, phase 3 trial.Lancet Oncol. 2022 Feb;23(2):209-219. doi: 10.1016/S1470-2045(21)00630-6. Epub 2022 Jan 14. Lancet Oncol. 2022. PMID: 35038429 Clinical Trial.
-
Switching administration of anti-PD-1 and anti-PD-L1 antibodies as immune checkpoint inhibitor rechallenge in individuals with advanced non-small cell lung cancer: Case series and literature review.Thorac Cancer. 2020 Jul;11(7):1927-1933. doi: 10.1111/1759-7714.13483. Epub 2020 May 18. Thorac Cancer. 2020. PMID: 32421224 Free PMC article.
-
Induction PD-1 inhibitor toripalimab plus chemotherapy followed by concurrent chemoradiotherapy and consolidation toripalimab for bulky locally advanced non-small-cell lung cancer: protocol for a randomized phase II trial (InTRist study).Front Immunol. 2024 Jan 15;14:1341584. doi: 10.3389/fimmu.2023.1341584. eCollection 2023. Front Immunol. 2024. PMID: 38288117 Free PMC article.
-
Immune Checkpoint Inhibition With Chemoradiotherapy in Stage III Non-small-cell Lung Cancer: A Systematic Review and Meta-analysis of Safety Results.Clin Lung Cancer. 2021 Mar;22(2):74-82. doi: 10.1016/j.cllc.2020.10.023. Epub 2020 Nov 12. Clin Lung Cancer. 2021. PMID: 33414053
-
Immune checkpoint-inhibitors and chemoradiation in stage III unresectable non-small cell lung cancer.Lung Cancer. 2019 Aug;134:259-267. doi: 10.1016/j.lungcan.2019.05.027. Epub 2019 May 29. Lung Cancer. 2019. PMID: 31319991 Review.
Cited by
-
Efficacy and safety of definitive chemoradiotherapy with or without induction immune checkpoint inhibitors in patients with stage III non-small cell lung cancer.Front Immunol. 2023 Nov 24;14:1281888. doi: 10.3389/fimmu.2023.1281888. eCollection 2023. Front Immunol. 2023. PMID: 38077319 Free PMC article.
-
Incidences of pneumonitis associated with the combination of radiotherapy and immune checkpoint inhibitors in lung cancer: a systematic review and meta-analysis.Front Oncol. 2025 Apr 17;15:1365966. doi: 10.3389/fonc.2025.1365966. eCollection 2025. Front Oncol. 2025. PMID: 40313247 Free PMC article.
-
Pre-Existing Interstitial Lung Abnormalities Are Independent Risk Factors for Interstitial Lung Disease during Durvalumab Treatment after Chemoradiotherapy in Patients with Locally Advanced Non-Small-Cell Lung Cancer.Cancers (Basel). 2022 Dec 17;14(24):6236. doi: 10.3390/cancers14246236. Cancers (Basel). 2022. PMID: 36551721 Free PMC article.
-
Characterising immune-related adverse events in different types of cancer among Chinese patients receiving immune checkpoint inhibitors.Sci Rep. 2024 Dec 28;14(1):30983. doi: 10.1038/s41598-024-82105-3. Sci Rep. 2024. PMID: 39730637 Free PMC article.
-
The application of bronchoscopy in the assessment of immune checkpoint inhibitor-related pneumonitis severity and recurrence.Sci Rep. 2024 Jul 25;14(1):17137. doi: 10.1038/s41598-024-66768-6. Sci Rep. 2024. PMID: 39060280 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70 (1): 7–30. - PubMed
-
- Yang P, Allen MS, Aubry MC et al Clinical features of 5,628 primary lung cancer patients: Experience at Mayo Clinic from 1997 to 2003. Chest 2005; 128 (1): 452–62. - PubMed
-
- Govindan R, Bogart J, Vokes EE. Locally advanced non‐small cell lung cancer: The past, present, and future. J Thorac Oncol 2008; 3 (8): 917–28. - PubMed
-
- Yamamoto N, Nakagawa K, Nishimura Y et al Phase III study comparing second‐ and third‐generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non‐small‐cell lung cancer: West Japan thoracic oncology group WJTOG0105. J Clin Oncol 2010; 28 (23): 3739–45. - PubMed
-
- Furuse K, Fukuoka M, Kawahara M et al Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non‐small‐cell lung cancer. J Clin Oncol 1999; 17 (9): 2692–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials


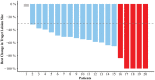
 , CR;
, CR;  , PR;
, PR;  , SD.
, SD.