Comparing Beta Cell Preservation Across Clinical Trials in Recent-Onset Type 1 Diabetes
- PMID: 32833543
- PMCID: PMC7757538
- DOI: 10.1089/dia.2020.0305
Comparing Beta Cell Preservation Across Clinical Trials in Recent-Onset Type 1 Diabetes
Abstract
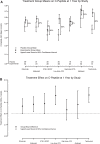
Several immunotherapies have demonstrated endogenous insulin preservation in recent-onset type 1 diabetes (T1D). We considered the primary results of rituximab, abatacept, teplizumab, alefacept, high-dose antithymocyte globulin (ATG), low-dose ATG, and low-dose ATG ± granulocyte-colony-stimulating factor trials in an attempt to rank the effectiveness of the agents studied. C-peptide 2-h area under the curve means were modeled using analysis of covariance. The experimental treatment group effect for each study, compared with its internal control, was estimated after adjusting for baseline C-peptide and age. Percentage increase in C-peptide over placebo and the absolute difference within study were calculated to compare and contrast effect size among interventions. Low-dose ATG (55% and 103%) and teplizumab (48% and 63%) ranked highest in C-peptide preservation at 1 and 2 years, respectively. Low-dose ATG and teplizumab show the greatest impact on C-peptide preservation among recent new-onset T1D studies; these should be further explored as core immunotherapies in the T1D prevention setting.
Keywords: Clinical trial; Immunotherapy; Intervention; Type 1 diabetes.
Conflict of interest statement
M.J.H. is on the scientific advisory board of SAb Biotherapeutics and received grant funding from Sanofi, and The Helmsley Charitable Trust to perform studies of low-dose ATG. MAA holds a patent on ATG plus G-CSF for the treatment of type 1 diabetes. There are no other conflicts of interest to disclose.
Figures

References
-
- Herold KC, Gitelman SE, Ehlers MR, et al. : Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 2013;62:3766–3774 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AI042288/AI/NIAID NIH HHS/United States
- U01 DK103282/DK/NIDDK NIH HHS/United States
- UL1 TR000114/TR/NCATS NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- U01 DK107013/DK/NIDDK NIH HHS/United States
- U01 DK061034/DK/NIDDK NIH HHS/United States
- U01 DK103180/DK/NIDDK NIH HHS/United States
- U01 DK106984/DK/NIDDK NIH HHS/United States
- U01 DK085453/DK/NIDDK NIH HHS/United States
- UC4 DK117009/DK/NIDDK NIH HHS/United States
- U01 DK085499/DK/NIDDK NIH HHS/United States
- U01 DK107014/DK/NIDDK NIH HHS/United States
- UC4 DK097835/DK/NIDDK NIH HHS/United States
- U01 DK085465/DK/NIDDK NIH HHS/United States
- U01 DK106994/DK/NIDDK NIH HHS/United States
- U01 DK085476/DK/NIDDK NIH HHS/United States
- U01 DK061010/DK/NIDDK NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U01 DK085466/DK/NIDDK NIH HHS/United States
- U01 DK103153/DK/NIDDK NIH HHS/United States
- U01 DK085504/DK/NIDDK NIH HHS/United States
- U01 DK106993/DK/NIDDK NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- UL1 RR024139/RR/NCRR NIH HHS/United States
- U01 DK061058/DK/NIDDK NIH HHS/United States
- UC4 DK106993/DK/NIDDK NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- U01 DK103266/DK/NIDDK NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- U01 DK061042/DK/NIDDK NIH HHS/United States
- U01 DK085461/DK/NIDDK NIH HHS/United States
- U01 DK085509/DK/NIDDK NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- UL1 TR000442/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
