Model-based cost-effectiveness estimates of testing strategies for diagnosing hepatitis C virus infection in Central and Western Africa
- PMID: 32833976
- PMCID: PMC7446873
- DOI: 10.1371/journal.pone.0238035
Model-based cost-effectiveness estimates of testing strategies for diagnosing hepatitis C virus infection in Central and Western Africa
Abstract
Background: Whereas 72% of hepatitis C virus (HCV)-infected people worldwide live in low- and middle-income countries (LMICs), only 6% of them have been diagnosed. Innovative technologies for HCV diagnosis provide opportunities for developing testing strategies more adapted to resource-constrained settings. However, studies about their economic feasibility in LMICs are lacking.
Methods: Adopting a health sector perspective in Cameroon, Cote-d'Ivoire, and Senegal, a decision tree model was developed to compare 12 testing strategies with the following characteristics: a one-step or two-step testing sequence, HCV-RNA or HCV core antigen as confirmative biomarker, laboratory or point-of-care (POC) tests, and venous blood samples or dried blood spots (DBS). Outcomes measures were the number of true positives (TPs), cost per screened individual, incremental cost-effectiveness ratios, and nationwide budget. Corresponding time horizon was immediate, and outcomes were accordingly not discounted. Detailed sensitivity analyses were conducted.
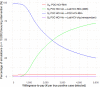
Findings: In the base-case, a two-step POC-based strategy including anti-HCV antibody (HCV-Ab) and HCV-RNA testing had the lowest cost, €8.18 per screened individual. Assuming a lost-to-follow-up rate after screening > 1.9%, a DBS-based laboratory HCV-RNA after HCV-Ab POC testing was the single un-dominated strategy, requiring an additional cost of €3653.56 per additional TP detected. Both strategies would require 8-25% of the annual public health expenditure of the study countries for diagnosing 30% of HCV-infected individuals. Assuming a seroprevalence > 46.9% or a cost of POC HCV-RNA < €7.32, a one-step strategy based on POC HCV-RNA dominated the two-step POC-based strategy but resulted in many more false-positive cases.
Conclusions: POC HCV-Ab followed by either POC- or DBS-based HCV-RNA testing would be the most cost-effective strategies in the study countries. Without a substantial increase in funding for health or a dramatic decrease in assay prices, HCV testing would constitute an economic barrier to the implementation of HCV elimination programs in LMICs.
Conflict of interest statement
K. Lacombe reports personal fees and non-financial support from GILEAD, personal fees and nonfinancial support from ABBVIE, personal fees and non-financial support from JANSSEN, grants, personal fees and non-financial support from MSD, outside the submitted work. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- WHO. Global hepatitis report, Web Annex B, WHO estimates of the prevalence and incidence of hepatitis C virus infection by WHO region, 2015 [Internet]. 2017 [cited 2017 Aug 3]. Available from: http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/
-
- WHO. Progress report on access to hepatitis C treatment [Internet]. 2018 [cited 2018 Sep 26]. Available from: http://www.who.int/hepatitis/publications/hep-c-access-report-2018/en/
-
- Tordrup D, Hutin Y, Stenberg K, Lauer JA, Hutton DW, Toy M, et al. Additional resource needs for viral hepatitis elimination through universal health coverage: projections in 67 low-income and middle-income countries, 2016–30. Lancet Glob Health. 2019. September 1;7(9):e1180–8. 10.1016/S2214-109X(19)30272-4 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

