Comparison of loop-mediated isothermal amplification (LAMP) and PCR for the diagnosis of infection with Trypanosoma brucei ssp. in equids in The Gambia
- PMID: 32833981
- PMCID: PMC7444819
- DOI: 10.1371/journal.pone.0237187
Comparison of loop-mediated isothermal amplification (LAMP) and PCR for the diagnosis of infection with Trypanosoma brucei ssp. in equids in The Gambia
Abstract
Introduction: Infection of equids with Trypanosoma brucei (T. brucei) ssp. is of socioeconomic importance across sub-Saharan Africa as the disease often progresses to cause fatal meningoencephalitis. Loop-mediated isothermal amplification (LAMP) has been developed as a cost-effective molecular diagnostic test and is potentially applicable for use in field-based laboratories.
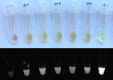
Part i: Threshold levels for T. brucei ssp. detection by LAMP were determined using whole equine blood specimens spiked with known concentrations of parasites. Results were compared to OIE antemortem gold standard of T. brucei-PCR (TBR-PCR).
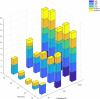
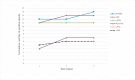
Results i: Threshold for detection of T. brucei ssp. on extracted DNA from whole blood was 1 parasite/ml blood for LAMP and TBR-PCR, and there was excellent agreement (14/15) between tests at high (1 x 103/ml) concentrations of parasites. Detection threshold was 100 parasites/ml using LAMP on whole blood (LWB). Threshold for LWB improved to 10 parasites/ml with detergent included. Performance was excellent for LAMP at high (1 x 103/ml) concentrations of parasites (15/15, 100%) but was variable at lower concentrations. Agreement between tests was weak to moderate, with the highest for TBR-PCR and LAMP on DNA extracted from whole blood (Cohen's kappa 0.95, 95% CI 0.64-1.00).
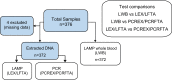
Part ii: A prospective cross-sectional study of working equids meeting clinical criteria for trypanosomiasis was undertaken in The Gambia. LAMP was evaluated against subsequent TBR-PCR.
Results ii: Whole blood samples from 321 equids in The Gambia were processed under field conditions. There was weak agreement between LWB and TBR-PCR (Cohen's kappa 0.34, 95% CI 0.19-0.49) but excellent agreement when testing CSF (100% agreement on 6 samples).
Conclusions: Findings support that LAMP is comparable to PCR when used on CSF samples in the field, an important tool for clinical decision making. Results suggest repeatability is low in animals with low parasitaemia. Negative samples should be interpreted in the context of clinical presentation.
Conflict of interest statement
LAMP kits and LAMP incubator for field use were provided free of charge by Joseph M N’dungu and FIND Diagnostics. These parties had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare that they have no competing interests.
Figures


Similar articles
-
Using detergent-enhanced LAMP for African trypanosome detection in human cerebrospinal fluid and implications for disease staging.PLoS Negl Trop Dis. 2019 Aug 19;13(8):e0007631. doi: 10.1371/journal.pntd.0007631. eCollection 2019 Aug. PLoS Negl Trop Dis. 2019. PMID: 31425540 Free PMC article.
-
Loop Mediated Isothermal Amplification for Detection of Trypanosoma brucei gambiense in Urine and Saliva Samples in Nonhuman Primate Model.Biomed Res Int. 2015;2015:867846. doi: 10.1155/2015/867846. Epub 2015 Oct 4. Biomed Res Int. 2015. PMID: 26504841 Free PMC article.
-
Diagnostic accuracy of loopamp Trypanosoma brucei detection kit for diagnosis of human African trypanosomiasis in clinical samples.PLoS Negl Trop Dis. 2013 Oct 17;7(10):e2504. doi: 10.1371/journal.pntd.0002504. eCollection 2013. PLoS Negl Trop Dis. 2013. PMID: 24147176 Free PMC article.
-
Loop-mediated isothermal amplification (LAMP): An advanced molecular point-of-care technique for the detection of Leishmania infection.PLoS Negl Trop Dis. 2019 Nov 7;13(11):e0007698. doi: 10.1371/journal.pntd.0007698. eCollection 2019 Nov. PLoS Negl Trop Dis. 2019. PMID: 31697673 Free PMC article. Review.
-
A pooled analysis of the LAMP assay for the detection of Neisseria meningitidis.BMC Infect Dis. 2020 Jul 20;20(1):525. doi: 10.1186/s12879-020-05250-w. BMC Infect Dis. 2020. PMID: 32689953 Free PMC article.
Cited by
-
Current and Future Advances in the Detection and Surveillance of Biosecurity-Relevant Equine Bacterial Diseases Using Loop-Mediated Isothermal Amplification (LAMP).Animals (Basel). 2023 Aug 18;13(16):2663. doi: 10.3390/ani13162663. Animals (Basel). 2023. PMID: 37627456 Free PMC article. Review.
-
Development and Assessment of a Multiple-Analysis System for Diagnosing Malaria and Other Blood Parasite Infections in Humans and Non-Human Primates.Diagnostics (Basel). 2025 Mar 4;15(5):620. doi: 10.3390/diagnostics15050620. Diagnostics (Basel). 2025. PMID: 40075867 Free PMC article.
-
Isothermal Nucleic Acid Amplification to Detect Infection Caused by Parasites of the Trypanosomatidae Family: A Literature Review and Opinion on the Laboratory to Field Applicability.Int J Mol Sci. 2022 Jul 7;23(14):7543. doi: 10.3390/ijms23147543. Int J Mol Sci. 2022. PMID: 35886895 Free PMC article. Review.
-
Extracellular Vesicles Derived from Trypanosomatids: The Key to Decoding Host-Parasite Communication.Int J Mol Sci. 2025 May 1;26(9):4302. doi: 10.3390/ijms26094302. Int J Mol Sci. 2025. PMID: 40362539 Free PMC article. Review.
References
-
- Sowe J, Gai B, Sumberg J, Gilbert E. Foaling and mortality of equines in the Gambia: a national survey In: Starkey P, Faye A, editors. Animal Traction for agricultural development. Wageningen: Technical Centre for Agricultural and Rural Cooperation; 1988. pp. 315–321.
-
- Upjohn M, Valette D. The Relationship Between Working Equids and Women in Developing Countries. Equine Vet J. 2014;46: 20–20. 10.1111/evj.12323_46 - DOI
-
- Pinchbeck GL, Morrison LJ, Tait A, Langford J, Meehan L, Jallow S, et al. Trypanosomosis in The Gambia: prevalence in working horses and donkeys detected by whole genome amplification and PCR, and evidence for interactions between trypanosome species. BMC Vet Res. 2008;4: 7 10.1186/1746-6148-4-7 - DOI - PMC - PubMed
-
- Faye D, Pereira de Almeida PJ, Goossens B, Osaer S, Ndao M, Berkvens D, et al. Prevalence and incidence of trypanosomosis in horses and donkeys in the Gambia. Vet Parasitol. 2001;101: 101–14. Available: http://www.ncbi.nlm.nih.gov/pubmed/11587839 10.1016/s0304-4017(01)00503-9 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

