Alpha-crystallin mutations alter lens metabolites in mouse models of human cataracts
- PMID: 32833997
- PMCID: PMC7446835
- DOI: 10.1371/journal.pone.0238081
Alpha-crystallin mutations alter lens metabolites in mouse models of human cataracts
Erratum in
-
Correction: Alpha-crystallin mutations alter lens metabolites in mouse models of human cataracts.PLoS One. 2020 Nov 20;15(11):e0242951. doi: 10.1371/journal.pone.0242951. eCollection 2020. PLoS One. 2020. PMID: 33216808 Free PMC article.
Abstract
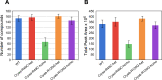
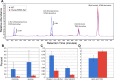
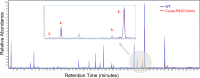
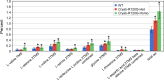
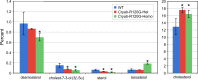
Cataracts are a major cause of blindness worldwide and commonly occur in individuals over 70 years old. Cataracts can also appear earlier in life due to genetic mutations. The lens proteins, αA- and αB-crystallins, are chaperone proteins that have important roles maintaining protein solubility to prevent cataract formation. Mutations in the CRYAA and CRYAB crystallin genes are associated with autosomal dominant early onset human cataracts. Although studies about the proteomic and genomic changes that occur in cataracts have been reported, metabolomics studies are very limited. Here, we directly investigated cataract metabolism using gas-chromatography-mass spectrometry (GC-MS) to analyze the metabolites in adult Cryaa-R49C and Cryab-R120G knock-in mouse lenses. The most abundant metabolites were myo-inositol, L-(+)-lactic acid, cholesterol, phosphate, glycerol phosphate, palmitic and 9-octadecenoic acids, α-D-mannopyranose, and β-D-glucopyranose. Cryaa-R49C knock-in mouse lenses had a significant decrease in the number of sugars and minor sterols, which occurred in concert with an increase in lactic acid. Cholesterol composition was unchanged. In contrast, Cryab-R120G knock-in lenses exhibited increased total amino acid content including valine, alanine, serine, leucine, isoleucine, glycine, and aspartic acid. Minor sterols, including cholest-7-en-3-ol and glycerol phosphate were decreased. These studies indicate that lenses from Cryaa-R49C and Cryab-R120G knock-in mice, which are models for human cataracts, have unique amino acid and metabolite profiles.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Probing the changes in gene expression due to α-crystallin mutations in mouse models of hereditary human cataract.PLoS One. 2018 Jan 16;13(1):e0190817. doi: 10.1371/journal.pone.0190817. eCollection 2018. PLoS One. 2018. PMID: 29338044 Free PMC article.
-
Changes in relative histone abundance and heterochromatin in αA-crystallin and αB-crystallin knock-in mutant mouse lenses.BMC Res Notes. 2020 Jul 2;13(1):315. doi: 10.1186/s13104-020-05154-7. BMC Res Notes. 2020. PMID: 32616056 Free PMC article.
-
Autophagy and UPR in alpha-crystallin mutant knock-in mouse models of hereditary cataracts.Biochim Biophys Acta. 2016 Jan;1860(1 Pt B):234-9. doi: 10.1016/j.bbagen.2015.06.001. Epub 2015 Jun 11. Biochim Biophys Acta. 2016. PMID: 26071686 Free PMC article.
-
Genetics of crystallins: cataract and beyond.Exp Eye Res. 2009 Feb;88(2):173-89. doi: 10.1016/j.exer.2008.10.011. Epub 2008 Nov 1. Exp Eye Res. 2009. PMID: 19007775 Review.
-
Effects of alpha-crystallin on lens cell function and cataract pathology.Curr Mol Med. 2009 Sep;9(7):887-92. doi: 10.2174/156652409789105598. Curr Mol Med. 2009. PMID: 19860667 Review.
Cited by
-
Generation of Lens Progenitor Cells and Lentoid Bodies from Pluripotent Stem Cells: Novel Tools for Human Lens Development and Ocular Disease Etiology.Cells. 2022 Nov 6;11(21):3516. doi: 10.3390/cells11213516. Cells. 2022. PMID: 36359912 Free PMC article. Review.
-
Oxysterol Compounds in Mouse Mutant αA- and αB-Crystallin Lenses Can Improve the Optical Properties of the Lens.Invest Ophthalmol Vis Sci. 2022 May 2;63(5):15. doi: 10.1167/iovs.63.5.15. Invest Ophthalmol Vis Sci. 2022. PMID: 35575904 Free PMC article.
-
Precise Detection of Cataracts with Specific High-Risk Factors by Layered Binary Co-Ionizers Assisted Aqueous Humor Metabolic Analysis.Adv Sci (Weinh). 2022 Jul;9(21):e2105905. doi: 10.1002/advs.202105905. Epub 2022 May 26. Adv Sci (Weinh). 2022. PMID: 35621284 Free PMC article.
-
Physiological and pathological functions of βB2-crystallins in multiple organs: a systematic review.Aging (Albany NY). 2021 Jun 11;13(11):15674-15687. doi: 10.18632/aging.203147. Epub 2021 Jun 11. Aging (Albany NY). 2021. PMID: 34118792 Free PMC article.
-
Survey of patient satisfaction after bilateral cataract surgery.Rom J Ophthalmol. 2022 Jan-Mar;66(1):36-40. doi: 10.22336/rjo.2022.9. Rom J Ophthalmol. 2022. PMID: 35531447 Free PMC article.
References
-
- Harrington V, McCall S, Huynh S, Srivastava K, Srivastava OP. Crystallins in water soluble-high molecular weight protein fractions and water insoluble protein fractions in aging and cataractous human lenses. Mol Vis. 2004;10:476–89. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

