Myo/Nog cells are nonprofessional phagocytes
- PMID: 32833999
- PMCID: PMC7446839
- DOI: 10.1371/journal.pone.0235898
Myo/Nog cells are nonprofessional phagocytes
Abstract
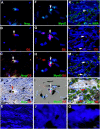
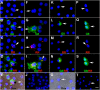
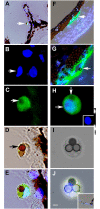
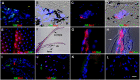
Myo/Nog cells were discovered in the chick embryo epiblast. Their expression of MyoD reflects a commitment to the skeletal muscle lineage and capacity to differentiate into myofibroblasts. Release of Noggin by Myo/Nog cells is essential for normal morphogenesis. Myo/Nog cells rapidly respond to wounding in the skin and eyes. In this report, we present evidence suggesting that Myo/Nog cells phagocytose tattoo ink in tissue sections of human skin and engulf cell corpses in cultures of anterior human lens tissue and magnetic beads injected into the anterior chamber of mice in vivo. Myo/Nog cells are distinct from macrophages in the skin and eyes indicated by the absence of labeling with an antibody to ionized calcium binding adaptor molecule 1. In addition to their primary roles as regulators of BMP signaling and progenitors of myofibroblasts, Myo/Nog cells behave as nonprofessional phagocytes defined as cells whose primary functions are unrelated to phagocytosis but are capable of engulfment.
Conflict of interest statement
There are no competing interests related to this manuscript.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

