Cell signaling model for arterial mechanobiology
- PMID: 32834001
- PMCID: PMC7470387
- DOI: 10.1371/journal.pcbi.1008161
Cell signaling model for arterial mechanobiology
Abstract
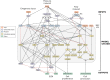
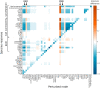
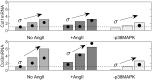
Arterial growth and remodeling at the tissue level is driven by mechanobiological processes at cellular and sub-cellular levels. Although it is widely accepted that cells seek to promote tissue homeostasis in response to biochemical and biomechanical cues-such as increased wall stress in hypertension-the ways by which these cues translate into tissue maintenance, adaptation, or maladaptation are far from understood. In this paper, we present a logic-based computational model for cell signaling within the arterial wall, aiming to predict changes in extracellular matrix turnover and cell phenotype in response to pressure-induced wall stress, flow-induced wall shear stress, and exogenous sources of angiotensin II, with particular interest in mouse models of hypertension. We simulate a number of experiments from the literature at both the cell and tissue level, involving single or combined inputs, and achieve high qualitative agreement in most cases. Additionally, we demonstrate the utility of this modeling approach for simulating alterations (in this case knockdowns) of individual nodes within the signaling network. Continued modeling of cellular signaling will enable improved mechanistic understanding of arterial growth and remodeling in health and disease, and will be crucial when considering potential pharmacological interventions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

