Change in exhaled nitric oxide during peanut challenge is related to severity of reaction
- PMID: 32834829
- PMCID: PMC7386245
- DOI: 10.1186/s13223-020-00464-8
Change in exhaled nitric oxide during peanut challenge is related to severity of reaction
Abstract
Background: Peanut allergy affects 3% of Australian children and has a higher risk of anaphylaxis than most food allergies. Predicting who is likely to develop anaphylaxis is still an inexact science. The fraction of exhaled nitric oxide (FeNO) shows promise as a biomarker involved in peanut allergy, as nitric oxide plays a role in inhibiting mast cell degranulation which is relevant in anaphylaxis, where mast cell degranulation plays a mediator role. The aim of this study was to assess the change in FeNO in children during peanut challenge.
Methods: Thirty-six children aged from 5 to 17 years were recruited for open-labelled peanut challenge. Participants had skin prick test to peanut performed, and serum collected for Ara h2 specific IgE and peanut specific IgE. FeNO was measured by portable device (NIOX VERO) prior to and throughout the peanut challenge.
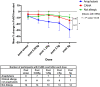
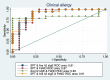
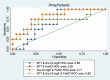
Results: When grouped according to reaction type at peanut challenge (anaphylaxis, clinical allergy not anaphylaxis and tolerant), there were significant differences in the mean change in FeNO measurement between the anaphylaxis group and the clinical allergy, not anaphylaxis group (p = 0.005), and between the anaphylaxis group and tolerant group (p < 0.0001).
Conclusions: FeNO decreased more significantly in those who subsequently developed anaphylaxis than in those with clinical allergy, not anaphylaxis or negative peanut challenge (tolerance). As a bedside test that can be used in children, it has potential for further research into mechanisms of anaphylaxis in food allergy and potentially assists in predicting an imminent anaphylactic reaction in some patients.Trial registration ClinicalTrials.gov: PEAnut Anaphylaxis Predictors (PEAAP) NCT02424136.
Keywords: Allergy; Anaphylaxis; Ara h2 sIgE; FeNO; Fraction exhaled nitric oxide; Peanut; Peanut sIgE; Skin prick test.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures

Similar articles
-
Reproducibility of serum IgE, Ara h2 skin prick testing and fraction of exhaled nitric oxide for predicting clinical peanut allergy in children.Allergy Asthma Clin Immunol. 2016 Aug 5;12:35. doi: 10.1186/s13223-016-0143-z. eCollection 2016. Allergy Asthma Clin Immunol. 2016. PMID: 27499764 Free PMC article.
-
The fraction of exhaled nitric oxide improves prediction of clinical allergic reaction to peanut challenge in children.Clin Exp Allergy. 2014 Mar;44(3):371-80. doi: 10.1111/cea.12258. Clin Exp Allergy. 2014. PMID: 24345088
-
Peanut allergy and allergic airways inflammation.Pediatr Allergy Immunol. 2010 Dec;21(8):1107-13. doi: 10.1111/j.1399-3038.2010.01071.x. Pediatr Allergy Immunol. 2010. PMID: 20561237
-
Low-grade disease activity in early life precedes childhood asthma and allergy.Dan Med J. 2016 Aug;63(8):B5272. Dan Med J. 2016. PMID: 27477800 Review.
-
Peanut-Induced Anaphylaxis in Children: A Literature Review.Cureus. 2022 Dec 26;14(12):e32946. doi: 10.7759/cureus.32946. eCollection 2022 Dec. Cureus. 2022. PMID: 36578844 Free PMC article. Review.
Cited by
-
Exhaled Nitric Oxide as Biomarker of Type 2 Diseases.Cells. 2023 Oct 25;12(21):2518. doi: 10.3390/cells12212518. Cells. 2023. PMID: 37947596 Free PMC article. Review.
References
-
- Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127(3):668–676.e1–2. - PubMed
-
- Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107(1):191–193. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

