Highly pathogenic coronaviruses: thrusting vaccine development in the spotlight
- PMID: 32834948
- PMCID: PMC7260574
- DOI: 10.1016/j.apsb.2020.05.009
Highly pathogenic coronaviruses: thrusting vaccine development in the spotlight
Abstract
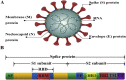
Coronaviruses (CoVs) are a large family of viruses that cause illness ranging from the common cold to more severe diseases such as Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS). Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) disease (COVID-19) has caused major public health crises. There have been more than 4,400,000 reported cases of COVID-2019 and more than 300,000 reported deaths to date (16/05/2020). SARS-CoV, MERS-CoV and SARS-CoV-2 have attracted widespread global attention due to their high infectivity and pathogenicity. To date, there is no specific treatment proven effective against these viral infectious diseases. Vaccination is considered one of the most effective strategies to prevent viral infections. Therefore, the development of effective vaccines against highly pathogenic coronaviruses is essential. In this review, we will briefly describe coronavirus vaccine design targets, summarize recent advances in the development of coronavirus vaccines, and highlight current adjuvants for improving the efficacy of coronavirus vaccines.
Keywords: Adjuvant; Coronaviruses; MERS-CoV; SARS-CoV; SARS-CoV-2; Vaccine.
© 2020 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures



References
-
- World Health Organization . 2020. Coronavirus disease (COVID-2019) situation reports.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio... [cited 2020 May 16th]. Available from:
-
- World Health Organization. Cumulative number of reported probable cases of severe acute respiratory syndrome (SARS) [cited 2020 March 30]. Available from: http://www.who.int/csr/sars/country/en/.
-
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV) [cited 2020 March 30]. Available from: https://www.who.int/emergencies/mers-cov/en/
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

