Anakinra for severe forms of COVID-19: a cohort study
- PMID: 32835245
- PMCID: PMC7259909
- DOI: 10.1016/S2665-9913(20)30164-8
Anakinra for severe forms of COVID-19: a cohort study
Abstract
Background: Coronaviruses can induce the production of interleukin (IL)-1β, IL-6, tumour necrosis factor, and other cytokines implicated in autoinflammatory disorders. It has been postulated that anakinra, a recombinant IL-1 receptor antagonist, might help to neutralise the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related hyperinflammatory state, which is considered to be one cause of acute respiratory distress among patients with COVID-19. We aimed to assess the off-label use of anakinra in patients who were admitted to hospital for severe forms of COVID-19 with symptoms indicative of worsening respiratory function.
Methods: The Ana-COVID study included a prospective cohort from Groupe Hospitalier Paris Saint-Joseph (Paris, France) and a historical control cohort retrospectively selected from the Groupe Hospitalier Paris Saint-Joseph COVID cohort, which began on March 18, 2020. Patients were included in the prospective cohort if they were aged 18 years or older and admitted to Groupe Hospitalier Paris Saint-Joseph with severe COVID-19-related bilateral pneumonia on chest x-ray or lung CT scan. The other inclusion criteria were either laboratory-confirmed SARS-CoV-2 or typical lung infiltrates on a lung CT scan, and either an oxygen saturation of 93% or less under oxygen 6 L/min or more, or aggravation (saturation ≤93% under oxygen 3 L/min) with a loss of 3% of oxygen saturation in ambient air over the previous 24 h. The historical control group of patients had the same inclusion criteria. Patients in the anakinra group were treated with subcutaneous anakinra (100 mg twice a day for 72 h, then 100 mg daily for 7 days) as well as the standard treatments at the institution at the time. Patients in the historical group received standard treatments and supportive care. The main outcome was a composite of either admission to the intensive care unit (ICU) for invasive mechanical ventilation or death. The main analysis was done on an intention-to-treat basis (including all patients in the anakinra group who received at least one injection of anakinra).
Findings: From March 24 to April 6, 2020, 52 consecutive patients were included in the anakinra group and 44 historical patients were identified in the Groupe Hospitalier Paris Saint-Joseph COVID cohort study. Admission to the ICU for invasive mechanical ventilation or death occurred in 13 (25%) patients in the anakinra group and 32 (73%) patients in the historical group (hazard ratio [HR] 0·22 [95% CI 0·11-0·41; p<0·0001). The treatment effect of anakinra remained significant in the multivariate analysis (HR 0·22 [95% CI 0·10-0·49]; p=0·0002). An increase in liver aminotransferases occurred in seven (13%) patients in the anakinra group and four (9%) patients in the historical group.
Interpretation: Anakinra reduced both need for invasive mechanical ventilation in the ICU and mortality among patients with severe forms of COVID-19, without serious side-effects. Confirmation of efficacy will require controlled trials.
Funding: Groupe Hospitalier Paris Saint-Joseph.
© 2020 Elsevier Ltd. All rights reserved.
Figures

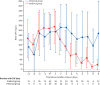
References
-
- Shrivastava G, León-Juárez M, García-Cordero J. Inflammasomes and its importance in viral infections. Immunol Res. 2016;64:1101–1117. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

