Characterization and Treatment of SARS-CoV-2 in Nasal and Bronchial Human Airway Epithelia
- PMID: 32835306
- PMCID: PMC7373044
- DOI: 10.1016/j.xcrm.2020.100059
Characterization and Treatment of SARS-CoV-2 in Nasal and Bronchial Human Airway Epithelia
Abstract
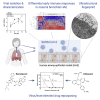
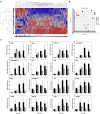
In the current COVID-19 pandemic context, proposing and validating effective treatments represents a major challenge. However, the scarcity of biologically relevant pre-clinical models of SARS-CoV-2 infection imposes a significant barrier for scientific and medical progress, including the rapid transition of potentially effective treatments to the clinical setting. We use reconstituted human airway epithelia to isolate and then characterize the viral infection kinetics, tissue-level remodeling of the cellular ultrastructure, and transcriptional early immune signatures induced by SARS-CoV-2 in a physiologically relevant model. Our results emphasize distinctive transcriptional immune signatures between nasal and bronchial HAE, both in terms of kinetics and intensity, hence suggesting putative intrinsic differences in the early response to SARS-CoV-2 infection. Most important, we provide evidence in human-derived tissues on the antiviral efficacy of remdesivir monotherapy and explore the potential of the remdesivir-diltiazem combination as an option worthy of further investigation to respond to the still-unmet COVID-19 medical need.
Keywords: COVID-19; SARS-CoV-2; cell ultrastructure remodeling; coronavirus; diltiazem; drug combination; innate immune response; remdesivir; repurposing.
© 2020 The Author(s).
Conflict of interest statement
A.P., B.P., T.J., A.T., O.T., and M.R.-C. are co-inventors of a patent application filed by INSERM, CNRS, Université Claude Bernard Lyon 1, and Signia Therapeutics for the repurposing of diltiazem for the treatment of SARS-CoV-2 infections (FR 20/02351). A.P., O.T., and M.R.-C. are co-founders of Signia Therapeutics SAS. S.C. is the founder of Epithelix, the developer and provider of MucilAir HAE. F.-X.L. has received funding for lectures from Gilead Sciences and Merck Sharp & Dohme France, and funding for travel to meetings from Merck Sharp & Dohme France, Astellas, and Eumedica. B.L. is the co-chair of the Global Influenza and RSV Initiative and the chair of the scientific committee of the Global Influenza Hospital Surveillance Network. B.L. received no personal remuneration for these activities. B.L. received travel grants to attend meetings by Abbott, Seegene, Sanofi, and bioMérieux. All of the other authors declare no competing interests.
Figures



References
-
- Hui D.S., I Azhar E., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., Mchugh T.D., Memish Z.A., Drosten C. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. - PMC - PubMed
-
- World Health Organization . 2020. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020.https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re...
-
- World Health Organization . 2020. Novel Coronavirus (2019-nCoV) Situation Report - 3, 23 January 2020.https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. 2020. Features, Evaluation and Treatment Coronavirus (COVID-19)https://www.ncbi.nlm.nih.gov/books/NBK554776/ - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

