A vitamin E blended highly cross-linked polyethylene acetabular cup results in less wear: 6-year results of a randomized controlled trial in 199 patients
- PMID: 32835560
- PMCID: PMC8023918
- DOI: 10.1080/17453674.2020.1807220
A vitamin E blended highly cross-linked polyethylene acetabular cup results in less wear: 6-year results of a randomized controlled trial in 199 patients
Abstract
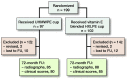
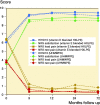
Background and purpose - Survivorship of total hip arthroplasty (THA) with the ultra-high molecular weight polyethylene (UHMWPE) monoblock cup has been limited due to periprosthetic osteolysis and aseptic loosening, secondary to wear of the UHMWPE. In response, a vitamin E blended highly cross-linked polyethylene (HXLPE) cup was developed. This study set out to compare the wear and clinical 6-year outcomes of vitamin E blended HXLPE with UHMWPE in an isoelastic monoblock cup in patients with hip osteoarthritis who underwent uncemented THA. The 2-year results have been reported previously. Patients and methods - For this randomized controlled trial 199 patients were included. 102 patients received the vitamin E blended HXLPE uncemented acetabular cup and 97 patients the uncemented UHMWPE monoblock cup. Clinical and radiographic parameters were obtained preoperatively, directly postoperatively, and at 3, 12, 24, and 72 months. Wear rates were compared using the femoral head penetration (FHP) rate. Results - 173 patients (87%) completed the 6-year follow-up. The mean NRS scores for rest pain, load pain, and patient satisfaction were 0.3 (SD 1), 0.6 (SD 1), and 8.6 (SD 1) respectively. The mean Harris Hip Score was 93 (SD 12). The FHP rate was lower in the vitamin E blended HXLPE cup (0.028 mm/year) compared with the UHMWPE cup (0.035 mm/year) (p = 0.002). No adverse reactions associated with the clinical application of vitamin E blended HXLPE were observed. 15 complications occurred, equally distributed between the two cups. The 6-year survival to revision rate was 98% for both cups. There was no aseptic loosening. Interpretation - This study shows the superior performance of the HXLPE blended with vitamin E acetabular cup with clinical and radiographic results similar to the UHMWPE acetabular cup.
Figures
Similar articles
-
2-year results of an RCT of 2 uncemented isoelastic monoblock acetabular components: lower wear rate with vitamin E blended highly cross-linked polyethylene compared to ultra-high molecular weight polyethylene.Acta Orthop. 2020 Jun;91(3):254-259. doi: 10.1080/17453674.2020.1730073. Epub 2020 Feb 26. Acta Orthop. 2020. PMID: 32098534 Free PMC article. Clinical Trial.
-
Does vitamin E-blended polyethylene reduce wear in primary total hip arthroplasty: a blinded randomised clinical trial.Int Orthop. 2017 Jun;41(6):1113-1118. doi: 10.1007/s00264-016-3320-2. Epub 2016 Nov 4. Int Orthop. 2017. PMID: 27815591 Clinical Trial.
-
Reduced wear in vitamin E-infused highly cross-linked polyethylene cups: 5-year results of a randomized controlled trial.Acta Orthop. 2021 Apr;92(2):151-155. doi: 10.1080/17453674.2020.1852785. Epub 2020 Dec 2. Acta Orthop. 2021. PMID: 33263447 Free PMC article. Clinical Trial.
-
Do monoblock cups improve survivorship, decrease wear, or reduce osteolysis in uncemented total hip arthroplasty?Clin Orthop Relat Res. 2013 Nov;471(11):3572-80. doi: 10.1007/s11999-013-3144-y. Epub 2013 Aug 3. Clin Orthop Relat Res. 2013. PMID: 23913339 Free PMC article.
-
Manufacturing, oxidation, mechanical properties and clinical performance of highly cross-linked polyethylene in total hip arthroplasty.Hip Int. 2018 Nov;28(6):573-583. doi: 10.1177/1120700018780677. Epub 2018 Jul 12. Hip Int. 2018. PMID: 29998768 Review.
Cited by
-
The concept of a cementless isoelastic monoblock cup made of highly cross-linked polyethylene infused with vitamin E: radiological analyses of migration and wear using EBRA and clinical outcomes at mid-term follow-up.BMC Musculoskelet Disord. 2021 Jan 23;22(1):107. doi: 10.1186/s12891-021-03981-8. BMC Musculoskelet Disord. 2021. PMID: 33485345 Free PMC article.
-
Head, acetabular liner composition, and rate of revision and wear in total hip arthroplasty: a Bayesian network meta-analysis.Sci Rep. 2023 Nov 21;13(1):20327. doi: 10.1038/s41598-023-47670-z. Sci Rep. 2023. PMID: 37989863 Free PMC article.
-
6-year follow-up on migration outcomes: a randomised clinical trial of cemented vitamin E-stabilised highly crosslinked versus standard polyethylene cup in total hip arthroplasty.Hip Int. 2024 Nov;34(6):695-700. doi: 10.1177/11207000241267971. Epub 2024 Sep 18. Hip Int. 2024. PMID: 39290199 Free PMC article. Clinical Trial.
-
In Vitro Wear of a Novel Vitamin E Crosslinked Polyethylene Lumbar Total Joint Replacement.Bioengineering (Basel). 2023 Oct 15;10(10):1198. doi: 10.3390/bioengineering10101198. Bioengineering (Basel). 2023. PMID: 37892928 Free PMC article.
-
Risk factors for liner wear and head migration in total hip arthroplasty: a systematic review.Sci Rep. 2023 Sep 20;13(1):15612. doi: 10.1038/s41598-023-42809-4. Sci Rep. 2023. PMID: 37730762 Free PMC article.
References
-
- Devane P A, Horne J G, Ashmore A, Mutimer J, Kim W, Stanley J.. Highly cross-linked polyethylene reduces wear and revision rates in total hip arthroplasty. J Bone Joint Surg 2017; 99(20): 1703–14. - PubMed
-
- Dorr L D, Wan Z, Shahrdar C, Sirianni L, Boutary M, Yun A.. Clinical performance of a Durasul highly cross-linked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg 2005; 87(8): 1816. - PubMed
-
- Dumbleton J H, Manley M T, Edidin A A.. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty 2002; 17(5): 649–61. - PubMed
-
- Galea V P, Connelly J W, Shareghi B, Kärrholm J, Sköldenberg O, Salemyr M, Laursen M B, Muratoglu O, Bragdon C, Malchau H.. Evaluation of in vivo wear of vitamin E-diffused highly crosslinked polyethylene at five years. Bone Joint J 2018; 100-B(12): 1592–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical