The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1α signalling pathway
- PMID: 32835596
- PMCID: PMC7469612
- DOI: 10.1080/21623945.2020.1807850
The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1α signalling pathway
Abstract
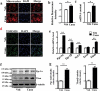
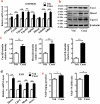
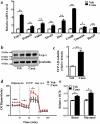
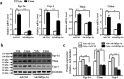
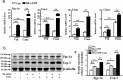
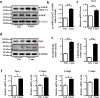
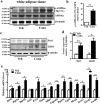
The diabetes medication canagliflozin (Cana) is a sodium glucose cotransporter 2 (SGLT2) inhibitor acting by increasing urinary glucose excretion and thus reducing hyperglycaemia. Cana treatment also reduces body weight. However, it remains unclear whether Cana could directly work on adipose tissue. In the present study, the pharmacological effects of Cana and the associated mechanism were investigated in adipocytes and mice. Stromal-vascular fractions (SVFs) were isolated from subcutaneous adipose tissue and differentiated into mature adipocytes. Our results show that Cana treatment directly increased cellular energy expenditure of adipocytes by inducing mitochondrial biogenesis independently of SGLT2 inhibition. Along with mitochondrial biogenesis, Cana also increased mitochondrial oxidative phosphorylation, fatty acid oxidation and thermogenesis. Mechanistically, Cana promoted mitochondrial biogenesis and function via an Adenosine monophosphate-activated protein kinase (AMPK) - silent information regulator 1 (Sirt1) - peroxisome proliferator-activated receptor γ coactivator-1α (Pgc-1α) signalling pathway. Consistently, in vivo study demonstrated that Cana increased AMPK phosphorylation and the expression of Sirt1 and Pgc-1α. The present study reveals a new therapeutic function for Cana in regulating energy homoeostasis.
Abbreviations: Ucp-1, uncoupling protein 1; cAMP, cyclic adenosine monophosphate; PKA, cAMP-dependent protein kinase A; SGLT, sodium glucose cotransporter; Cana, canagliflozin; T2DM: type 2 diabetes; Veh, vehicle; Pgc-1α, peroxisome proliferator-activated receptor γ coactivator-1α; SVFs, stromal-vascular fractions; FBS, bovine serum; Ad, adenovirus; mtDNA, mitochondrial DNA; COX2, cytochrome oxidase subunit 2; RT-PCR, real-time PCR; SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis; Prdm16, PR domain zinc finger protein 16; Cidea, cell death inducing DFFA-like effector A; Pgc-1β, peroxisome proliferator-activated receptor γ coactivator-1β; NRF1, nuclear respiratory factor 1; Tfam, mitochondrial transcription factor A; OXPHOS, oxidative phosphorylation; FAO, fatty acid oxidation; AMPK, Adenosine monophosphate-activated protein kinase; p-AMPK, phosphorylated AMPK; Sirt1, silent information regulator 1; mTOR, mammalian target of rapamycin; WAT, white adipose tissue; Fabp4, fatty acid binding protein 4; Lpl, lipoprotein lipase; Slc5a2, solute carrier family 5 member 2; ERRα, oestrogen related receptor α; Uqcrc2, ubiquinol-cytochrome c reductase core protein 2; Uqcrfs1, ubiquinol-cytochrome c reductase, Rieske iron-sulphur polypeptide 1; Cox4, cytochrome c oxidase subunit 4; Pparα, peroxisome proliferator activated receptor α; NAD+, nicotinamide adenine dinucleotide; Dio2, iodothyronine deiodinase 2; Tmem26, transmembrane protein 26; Hoxa9, homeobox A9; FCCP, carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone; Rot/AA, rotenone/antimycin A; OCR, oxygen consumption rate; Pparγ, peroxisome proliferator activated receptor γ; C/ebp, CCAAT/enhancer binding protein; LKB1, liver kinase B1; AUC, area under the cure; Vd, apparent volume of distribution.
Keywords: Canagliflozin; Pgc-1α; adipocytes; energy homoeostasis; mitochondrial biogenesis.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures

References
-
- Wai T, Langer T.. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab. 2016;27(2):105–117. - PubMed
-
- Deveaud C, Beauvoit B, Salin B, et al. Regional differences in oxidative capacity of rat white adipose tissue are linked to the mitochondrial content of mature adipocytes. Mol Cell Biochem. 2004;267:157–166. - PubMed
-
- Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
