Development and evaluation of deep learning-based segmentation of histologic structures in the kidney cortex with multiple histologic stains
- PMID: 32835732
- PMCID: PMC8414393
- DOI: 10.1016/j.kint.2020.07.044
Development and evaluation of deep learning-based segmentation of histologic structures in the kidney cortex with multiple histologic stains
Abstract
The application of deep learning for automated segmentation (delineation of boundaries) of histologic primitives (structures) from whole slide images can facilitate the establishment of novel protocols for kidney biopsy assessment. Here, we developed and validated deep learning networks for the segmentation of histologic structures on kidney biopsies and nephrectomies. For development, we examined 125 biopsies for Minimal Change Disease collected across 29 NEPTUNE enrolling centers along with 459 whole slide images stained with Hematoxylin & Eosin (125), Periodic Acid Schiff (125), Silver (102), and Trichrome (107) divided into training, validation and testing sets (ratio 6:1:3). Histologic structures were manually segmented (30048 total annotations) by five nephropathologists. Twenty deep learning models were trained with optimal digital magnification across the structures and stains. Periodic Acid Schiff-stained whole slide images yielded the best concordance between pathologists and deep learning segmentation across all structures (F-scores: 0.93 for glomerular tufts, 0.94 for glomerular tuft plus Bowman's capsule, 0.91 for proximal tubules, 0.93 for distal tubular segments, 0.81 for peritubular capillaries, and 0.85 for arteries and afferent arterioles). Optimal digital magnifications were 5X for glomerular tuft/tuft plus Bowman's capsule, 10X for proximal/distal tubule, arteries and afferent arterioles, and 40X for peritubular capillaries. Silver stained whole slide images yielded the worst deep learning performance. Thus, this largest study to date adapted deep learning for the segmentation of kidney histologic structures across multiple stains and pathology laboratories. All data used for training and testing and a detailed online tutorial will be publicly available.
Keywords: computerized morphologic assessment; deep learning; digital pathology; kidney histologic primitives; large-scale tissue interrogation; renal biopsy interpretation.
Copyright © 2020 International Society of Nephrology. All rights reserved.
Figures

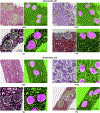



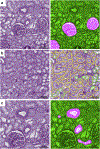





Comment in
-
Multistain segmentation of renal histology: first steps toward artificial intelligence-augmented digital nephropathology.Kidney Int. 2021 Jan;99(1):17-19. doi: 10.1016/j.kint.2020.08.025. Kidney Int. 2021. PMID: 33390226
References
-
- Bandari J, Fuller TW, Ii RMT, D’Agostino LA. Renal biopsy for medical renal disease: indications and contraindications. Can J Urol. 2016;23: 8121–8126. - PubMed
-
- Oni L, Beresford MW, Witte D, et al.Inter-observer variability of the histological classification of lupus glomerulonephritis in children. Lupus. 2017;26:1205–1211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

