Nuclear protein 1 imparts oncogenic potential and chemotherapeutic resistance in cancer
- PMID: 32835767
- PMCID: PMC7958295
- DOI: 10.1016/j.canlet.2020.08.019
Nuclear protein 1 imparts oncogenic potential and chemotherapeutic resistance in cancer
Abstract
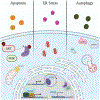
Nuclear protein 1 (NUPR1) also known as p8 and candidate of metastasis 1 (COM1) functions as a transcriptional regulator, and plays a role in cell cycle, DNA damage response, apoptosis, autophagy, and chromatin remodeling in response to various cellular stressors. Since it was first suggested to contribute to cancer development and progression in 1999, a number of studies have sought to reveal its function. However, NUPR1 and its biological relevance in cancer have proven difficult to pinpoint. Based on evidence of NUPR1 expression in cancers, its function extends from carcinogenesis and tumorigenesis to metastasis and chemotherapeutic resistance. A tumor suppressive function of NUPR1 has also been documented in multiple cancers. By and large, literature involving NUPR1 and cancer is confined to pancreatic and breast cancers, yet significant progress has been made with respect to NUPR1 expression and its function in lung, colorectal, blood, and prostate cancers, among others. Recent evidence strongly supports the notion that NUPR1 is key in chemotherapeutic resistance by mediating both anti-apoptotic activity and autophagy when challenged with anti-cancer compounds. Therefore, it is of significant importance to understand the broad range of molecular functions directed by NUPR1. In this review, NUPR1 expression and its role in breast, lung, and colorectal cancer development and progression will be addressed.
Keywords: Breast cancer; Chemotherapeutic resistance; Colorectal cancer; Lung cancer; NUPR1.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures

Similar articles
-
NUPR1 and its potential role in cancer and pathological conditions (Review).Int J Oncol. 2021 May;58(5):21. doi: 10.3892/ijo.2021.5201. Epub 2021 Mar 24. Int J Oncol. 2021. PMID: 33760183 Review.
-
The role of NUPR1 in response to stress and cancer development.Toxicol Appl Pharmacol. 2022 Nov 1;454:116244. doi: 10.1016/j.taap.2022.116244. Epub 2022 Sep 15. Toxicol Appl Pharmacol. 2022. PMID: 36116561 Review.
-
NUPR1 interacts with p53, transcriptionally regulates p21 and rescues breast epithelial cells from doxorubicin-induced genotoxic stress.Curr Cancer Drug Targets. 2008 Aug;8(5):421-30. doi: 10.2174/156800908785133196. Curr Cancer Drug Targets. 2008. PMID: 18690848
-
Emerging role of nuclear protein 1 (NUPR1) in cancer biology.Cancer Metastasis Rev. 2009 Jun;28(1-2):225-32. doi: 10.1007/s10555-009-9183-x. Cancer Metastasis Rev. 2009. PMID: 19153668 Review.
-
NUPR1 maintains autolysosomal efflux by activating SNAP25 transcription in cancer cells.Autophagy. 2018;14(4):654-670. doi: 10.1080/15548627.2017.1338556. Epub 2017 Dec 31. Autophagy. 2018. PMID: 29130426 Free PMC article.
Cited by
-
Single-cell atlas profiling revealed cellular characteristics and dynamic changes after PD-1 blockade therapy of brain metastases from laryngeal squamous cell carcinoma.Mol Cell Biochem. 2025 Apr;480(4):2377-2400. doi: 10.1007/s11010-024-05064-3. Epub 2024 Aug 1. Mol Cell Biochem. 2025. PMID: 39085744 Free PMC article.
-
NUPR1 Promotes Radioresistance in Colorectal Cancer Cells by Inhibiting Ferroptosis.J Cell Mol Med. 2025 Apr;29(7):e70519. doi: 10.1111/jcmm.70519. J Cell Mol Med. 2025. PMID: 40176685 Free PMC article.
-
Induction of NUPR1 and AP‑1 contributes to the carcinogenic potential of nickel.Oncol Rep. 2021 Apr;45(4):41. doi: 10.3892/or.2021.7992. Epub 2021 Mar 2. Oncol Rep. 2021. PMID: 33649793 Free PMC article.
-
DeepST: identifying spatial domains in spatial transcriptomics by deep learning.Nucleic Acids Res. 2022 Dec 9;50(22):e131. doi: 10.1093/nar/gkac901. Nucleic Acids Res. 2022. PMID: 36250636 Free PMC article.
-
Vismodegib Potentiates Marine Antimicrobial Peptide Tilapia Piscidin 4-Induced Cytotoxicity in Human Non-Small Cell Lung Cancer Cells.Probiotics Antimicrob Proteins. 2024 May 14. doi: 10.1007/s12602-024-10282-8. Online ahead of print. Probiotics Antimicrob Proteins. 2024. PMID: 38743208
References
-
- Bratland A, Risberg K, Maelandsmo GM, Gützkow KB, Olsen OE, Moghaddam A, … Ree AH (2000). Expression of a novel factor, com1, is regulated by 1,25-dihydroxyvitamin D3 in breast cancer cells. Cancer Res, 60(19), 5578–5583. Retrieved from https://cancerres.aacrjournals.org/content/canres/60/19/5578.full.pdf - PubMed
-
- Brünner N, Frandsen TL, Holst-Hansen C, Bei M, Thompson EW, Wakeling AE, … Clarke R (1993). MCF7/LCC2: a 4-hydroxytamoxifen resistant human breast cancer variant that retains sensitivity to the steroidal antiestrogen ICI 182,780. Cancer Res, 53(14), 3229–3232. Retrieved from https://cancerres.aacrjournals.org/content/canres/53/14/3229.full.pdf - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

