The ageing kidney: Molecular mechanisms and clinical implications
- PMID: 32835891
- PMCID: PMC7595250
- DOI: 10.1016/j.arr.2020.101151
The ageing kidney: Molecular mechanisms and clinical implications
Abstract
As human life expectancy keeps increasing, ageing populations present a growing challenge for clinical practices. Human ageing is associated with molecular, structural, and functional changes in a variety of organ systems, including the kidney. During the ageing process, the kidney experiences progressive functional decline as well as macroscopic and microscopic histological alterations, which are accentuated by systemic comorbidities like hypertension and diabetes mellitus, or by preexisting or underlying kidney diseases. Although ageing per se does not cause kidney injury, physiologic changes associated with normal ageing processes are likely to impair the reparative capacity of the kidney and thus predispose older people to acute kidney disease, chronic kidney disease and other renal diseases. Mechanistically, cell senescence plays a key role in renal ageing, involving a number of cellular signaling mechanisms, many of which may be harnessed as international targets for slowing or even reversing kidney ageing. This review summarizes the clinical characteristics of renal ageing, highlights the latest progresses in deciphering the role of cell senescence in renal ageing, and envisages potential interventional strategies and novel therapeutic targets for preventing or improving renal ageing in the hope of maintaining long-term kidney health and function across the life course.
Keywords: Glomeruli; Kidney diseases; Kidney transplantation; Nephrosclerosis; Renal tubules; Senescence.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
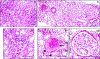
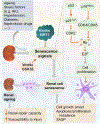
Figures


References
-
- Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil J, 2013. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 15, 978–990. - PMC - PubMed
-
- Administration for Community Living, 2019. 2018 profile of older Americans U.S. Department of Health and Human Services.
-
- Al-Douahji M, Brugarolas J, Brown PA, Stehman-Breen CO, Alpers CE, Shankland SJ, 1999. The cyclin kinase inhibitor p21WAF1/CIP1 is required for glomerular hypertrophy in experimental diabetic nephropathy. Kidney Int 56, 1691–1699. - PubMed
-
- Al-Said J, O’Neill WC, 2003. Reduced kidney size in patients with simple renal cysts. Kidney Int 64, 1059–1064. - PubMed
-
- Al-Said J, Brumback MA, Moghazi S, Baumgarten DA, O’Neill WC, 2004. Reduced renal function in patients with simple renal cysts. Kidney Int 65, 2303–2308. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

