Hemodynamic and Systemic Effects of Albumin in Patients with Advanced Liver Disease
- PMID: 32837825
- PMCID: PMC7326530
- DOI: 10.1007/s11901-020-00521-1
Hemodynamic and Systemic Effects of Albumin in Patients with Advanced Liver Disease
Abstract
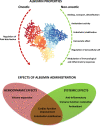
Purpose of review: Albumin administration is recommended to prevent or treat specific complications of decompensated cirrhosis based on its capacity to expand plasma volume. However, the molecule also has many other biological properties that are unrelated to the oncotic activity. The purpose of this review is to examine the hemodynamic and systemic effects of albumin administration in patients with decompensated cirrhosis.
Recent findings: Besides plasma expansion, albumin appears to act against inflammation, facilitate immunocompetence, and improve cardiac and endothelial function, thus antagonizing critical steps in the pathophysiological cascade underlying decompensated cirrhosis.
Summary: Increasing knowledge of the pathophysiological mechanisms of the disease, as well the pleiotropic properties of the molecule, provides the rationale for considering albumin as a multi-target disease-modifying agent in decompensated cirrhosis. Both oncotic and non-oncotic properties likely concur with the clinical benefits of long-term albumin administration recently demonstrated in these patients.
Keywords: Albumin; Ascites; Circulatory dysfunction; Liver cirrhosis; Systemic inflammation.
© The Author(s) 2020.
Conflict of interest statement
Conflict of InterestMT and GZ are members of the speakers’ bureau for Grifols SA and Octapharma AG. MB is a member of the speakers’ bureau for Octapharma AG. PC is a member of the speakers’ bureau for Grifols SA, Octapharma AG, Takeda SA, acts in advisory boards for Grifols SA, and has a research grant from Octapharma AG. AA has no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials