Systematic and Statistical Review of Coronavirus Disease 19 Treatment Trials
- PMID: 32838169
- PMCID: PMC7361001
- DOI: 10.1007/s42399-020-00399-6
Systematic and Statistical Review of Coronavirus Disease 19 Treatment Trials
Abstract
The following systematic review and meta-analysis compile the current data regarding human controlled COVID-19 treatment trials. An electronic search of the literature compiled studies pertaining to human controlled treatment trials with COVID-19. Medications assessed included lopinavir/ritonavir, arbidol, hydroxychloroquine, tocilizumab, favipiravir, heparin, and dexamethasone. Statistical analyses were performed for common viral clearance endpoints whenever possible. Lopinavir/ritonavir showed no significant effect on viral clearance for COVID-19 cases (OR 0.95 [95% CI 0.50-1.83]). Hydroxychloroquine also showed no significant effect on COVID-19 viral clearance rates (OR 2.16 [95% CI 0.80-5.84]). Arbidol showed no 7-day (OR 1.63 [95% CI 0.76-3.50]) or 14-day viral (OR 5.37 [95% CI 0.35-83.30]) clearance difference compared to lopinavir/ritonavir. Review of literature showed no significant clinical improvement with lopinavir/ritonavir, arbidol, hydroxychloroquine, or remdesivir. Tocilizumab showed mixed results regarding survival. Favipiravir showed quicker symptom improvement compared to lopinavir/ritonavir and arbidol. Heparin and dexamethasone showed improvement with severe COVID-19 cases requiring supplemental oxygenation. Current medications do not show significant effect on COVID-19 viral clearance rates. Tocilizumab showed mixed results regarding survival. Favipiravir shows favorable results compared to other tested medications. Heparin and dexamethasone show benefit especially for severe COVID-19 cases.
Keywords: Arbidol; COVID-19; Coronavirus; Dexamethasone; Favipiravir; Heparin; Hydroxychloroquine; Lopinavir/ritonavir; Remdesivir; SARS-CoV2; Tocilizumab; Treatment; Trials.
© Springer Nature Switzerland AG 2020.
Conflict of interest statement
Conflict of InterestThe authors declare that they have no conflict of interest.
Figures
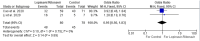

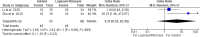
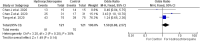
Similar articles
-
Effectiveness of Remdesivir, Lopinavir/Ritonavir, and Favipiravir for COVID-19 Treatment: A Systematic Review.Int J Gen Med. 2021 Nov 23;14:8557-8571. doi: 10.2147/IJGM.S332458. eCollection 2021. Int J Gen Med. 2021. PMID: 34849001 Free PMC article. Review.
-
Safety and efficacy of lopinavir/ritonavir combination in COVID-19: A systematic review, meta-analysis, and meta-regression analysis.Indian J Pharmacol. 2020 Jul-Aug;52(4):313-323. doi: 10.4103/ijp.IJP_627_20. Indian J Pharmacol. 2020. PMID: 33078733 Free PMC article.
-
Meta-analysis of arbidol versus lopinavir/ritonavir in the treatment of coronavirus disease 2019.J Med Virol. 2022 Apr;94(4):1513-1522. doi: 10.1002/jmv.27481. Epub 2021 Dec 6. J Med Virol. 2022. PMID: 34837230 Free PMC article.
-
Lopinavir-ritonavir alone or combined with arbidol in the treatment of 73 hospitalized patients with COVID-19: A pilot retrospective study.Int J Clin Pharmacol Ther. 2021 May;59(5):378-385. doi: 10.5414/CP203861. Int J Clin Pharmacol Ther. 2021. PMID: 33624583
-
Lopinavir-ritonavir versus hydroxychloroquine for viral clearance and clinical improvement in patients with mild to moderate coronavirus disease 2019.Korean J Intern Med. 2021 Mar;36(Suppl 1):S253-S263. doi: 10.3904/kjim.2020.224. Epub 2020 Jun 16. Korean J Intern Med. 2021. PMID: 32536150 Free PMC article.
Cited by
-
Scoping insight on antiviral drugs against COVID-19.Arab J Chem. 2021 Oct;14(10):103385. doi: 10.1016/j.arabjc.2021.103385. Epub 2021 Aug 16. Arab J Chem. 2021. PMID: 34909060 Free PMC article. Review.
-
Palliative Care for Patients on Extracorporeal Membrane Oxygenation for COVID-19 Infection.Am J Hosp Palliat Care. 2021 Jul;38(7):854-860. doi: 10.1177/10499091211001009. Epub 2021 Mar 9. Am J Hosp Palliat Care. 2021. PMID: 33685240 Free PMC article.
-
Dexamethasone for the Treatment of Coronavirus Disease (COVID-19): a Review.SN Compr Clin Med. 2020;2(12):2637-2646. doi: 10.1007/s42399-020-00610-8. Epub 2020 Oct 31. SN Compr Clin Med. 2020. PMID: 33163859 Free PMC article. Review.
-
The Outcome of Hydroxychloroquine in Patients Treated for COVID-19: Systematic Review and Meta-Analysis.Can Respir J. 2020 Oct 13;2020:4312519. doi: 10.1155/2020/4312519. eCollection 2020. Can Respir J. 2020. PMID: 33082891 Free PMC article.
-
Active site prediction of phosphorylated SARS-CoV-2 N-Protein using molecular simulation.Inform Med Unlocked. 2022;29:100889. doi: 10.1016/j.imu.2022.100889. Epub 2022 Feb 21. Inform Med Unlocked. 2022. PMID: 35224174 Free PMC article.
References
-
- World Health Organization. Coronavirus disease 2019 (COVID-19) situation report – 36. 2020.
-
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. - DOI - PMC - PubMed
-
- Li Y, Xie Z, Lin W, Cai W, Wen C, Guan Y, et al. An exploratory randomized, controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID-19 (ELACOI). MedRxiv. 2020. - PubMed
-
- Ye XT, Luo YL, Xia QF, Sun QF, Ding JG, Zhou Y, et al. Clinical efficacy of lopinavir/ritonavir in the treatment of coronavirus disease 2019. Eur Rev Med Pharmacol Sci. 2020;24:3390–3396. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
