Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study
- PMID: 32838323
- PMCID: PMC7428303
- DOI: 10.1016/S2665-9913(20)30277-0
Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study
Abstract
Background: Tocilizumab, a monoclonal antibody directed against the interleukin-6 receptor, has been proposed to mitigate the cytokine storm syndrome associated with severe COVID-19. We aimed to investigate the association between tocilizumab exposure and hospital-related mortality among patients requiring intensive care unit (ICU) support for COVID-19.
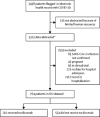
Methods: We did a retrospective observational cohort study at 13 hospitals within the Hackensack Meridian Health network (NJ, USA). We included patients (aged ≥18 years) with laboratory-confirmed COVID-19 who needed support in the ICU. We obtained data from a prospective observational database and compared outcomes in patients who received tocilizumab with those who did not. We applied a multivariable Cox model with propensity score matching to reduce confounding effects. The primary endpoint was hospital-related mortality. The prospective observational database is registered on ClinicalTrials.gov, NCT04347993.
Findings: Between March 1 and April 22, 2020, 764 patients with COVID-19 required support in the ICU, of whom 210 (27%) received tocilizumab. Factors associated with receiving tocilizumab were patients' age, gender, renal function, and treatment location. 630 patients were included in the propensity score-matched population, of whom 210 received tocilizumab and 420 did not receive tocilizumab. 358 (57%) of 630 patients died, 102 (49%) who received tocilizumab and 256 (61%) who did not receive tocilizumab. Overall median survival from time of admission was not reached (95% CI 23 days-not reached) among patients receiving tocilizumab and was 19 days (16-26) for those who did not receive tocilizumab (hazard ratio [HR] 0·71, 95% CI 0·56-0·89; p=0·0027). In the primary multivariable Cox regression analysis with propensity matching, an association was noted between receiving tocilizumab and decreased hospital-related mortality (HR 0·64, 95% CI 0·47-0·87; p=0·0040). Similar associations with tocilizumab were noted among subgroups requiring mechanical ventilatory support and with baseline C-reactive protein of 15 mg/dL or higher.
Interpretation: In this observational study, patients with COVID-19 requiring ICU support who received tocilizumab had reduced mortality. Results of ongoing randomised controlled trials are awaited.
Funding: None.
© 2020 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Tocilizumab in Treatment for Patients With COVID-19.JAMA Intern Med. 2021 Jul 1;181(7):1017-1018. doi: 10.1001/jamainternmed.2021.0392. JAMA Intern Med. 2021. PMID: 33818614 No abstract available.
References
-
- Johns Hopkins University of Medicine COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) Aug 13, 2020. https://coronavirus.jhu.edu/map.html
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

