Point of Care Nucleic Acid Testing for SARS-CoV-2 in Hospitalized Patients: A Clinical Validation Trial and Implementation Study
- PMID: 32838340
- PMCID: PMC7362826
- DOI: 10.1016/j.xcrm.2020.100062
Point of Care Nucleic Acid Testing for SARS-CoV-2 in Hospitalized Patients: A Clinical Validation Trial and Implementation Study
Abstract
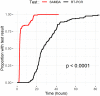
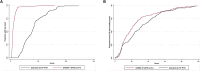
There is an urgent need for rapid SARS-CoV-2 testing in hospitals to limit nosocomial spread. We report an evaluation of point of care (POC) nucleic acid amplification testing (NAAT) in 149 participants with parallel combined nasal and throat swabbing for POC versus standard lab RT-PCR testing. Median time to result is 2.6 (IQR 2.3-4.8) versus 26.4 h (IQR 21.4-31.4, p < 0.001), with 32 (21.5%) positive and 117 (78.5%) negative. Cohen's κ correlation between tests is 0.96 (95% CI 0.91-1.00). When comparing nearly 1,000 tests pre- and post-implementation, the median time to definitive bed placement from admission is 23.4 (8.6-41.9) versus 17.1 h (9.0-28.8), p = 0.02. Mean length of stay on COVID-19 "holding" wards is 58.5 versus 29.9 h (p < 0.001). POC testing increases isolation room availability, avoids bed closures, allows discharge to care homes, and expedites access to hospital procedures. POC testing could mitigate the impact of COVID-19 on hospital systems.
Keywords: COVID-19; POC; SARS-CoV-2; diagnostic test; infection control; nosocomial; point of care.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Johns Hopkins University School of Medicine Coronavirus Resource Center COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) 2020. https://coronavirus.jhu.edu/map.html
-
- Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K., Liao P., Qiu J.F., Lin Y., Cai X.F. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26:845–848. - PubMed
-
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

