Safety and COVID-19 Symptoms in Individuals Recently Vaccinated with BCG: a Retrospective Cohort Study
- PMID: 32838341
- PMCID: PMC7405881
- DOI: 10.1016/j.xcrm.2020.100073
Safety and COVID-19 Symptoms in Individuals Recently Vaccinated with BCG: a Retrospective Cohort Study
Abstract
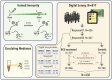
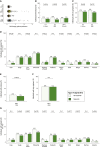
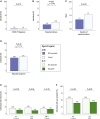
Bacille Calmette-Guérin (BCG) induces long-term boosting of innate immunity, termed trained immunity, and decreases susceptibility to respiratory tract infections. BCG vaccination trials for reducing SARS-CoV-2 infection are underway, but concerns have been raised regarding the potential harm of strong innate immune responses. To investigate the safety of BCG vaccination, we retrospectively assessed coronavirus disease 2019 (COVID-19) and related symptoms in three cohorts of healthy volunteers who either received BCG in the last 5 years or did not. BCG vaccination is not associated with increased incidence of symptoms during the COVID-19 outbreak in the Netherlands. Our data suggest that BCG vaccination might be associated with a decrease in the incidence of sickness during the COVID-19 pandemic (adjusted odds ratio [AOR] 0.58, p < 0.05), and lower incidence of extreme fatigue. In conclusion, recent BCG vaccination is safe, and large randomized trials are needed to reveal if BCG reduces the incidence and/or severity of SARS-CoV-2 infection.
Keywords: BCG; Bacille Calmette-Guérin; COVID-19; SARS-CoV-2; non-specific effects; off-target effects; trained immunity.
© 2020 The Author(s).
Conflict of interest statement
M.G.N. and L.A.B.J. are scientific founders of TTxD.
Figures



References
-
- World Health Organization . 2020. WHO Timeline-COVID-19.https://www.who.int/news-room/detail/27-04-2020-who-timeline---covid-19
-
- World Health Organization . 2020. Coronavirus disease (COVID-19): situation report-103.https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- Aaby P., Roth A., Ravn H., Napirna B.M., Rodrigues A., Lisse I.M., Stensballe L., Diness B.R., Lausch K.R., Lund N. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 2011;204:245–252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

