Outcomes of Hydroxychloroquine Usage in United States Veterans Hospitalized with COVID-19
- PMID: 32838355
- PMCID: PMC7274588
- DOI: 10.1016/j.medj.2020.06.001
Outcomes of Hydroxychloroquine Usage in United States Veterans Hospitalized with COVID-19
Abstract
Background: Despite limited and conflicting evidence, hydroxychloroquine, alone or in combination with azithromycin, is widely used in COVID-19 therapy.
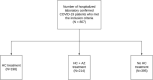
Methods: We performed a retrospective study of electronic health records of patients hospitalized with confirmed SARS-CoV-2 infection in US Veterans Health Administration medical centers between March 9, 2020 and April 29, 2020. Patients hospitalized within 24 h of diagnosis were classified based on their exposure to hydroxychloroquine alone (HC) or with azithromycin (HC+AZ) or no HC as treatments. The primary outcomes were mortality and use of mechanical ventilation.
Findings: A total of 807 patients were evaluated. Compared to the no HC group, after propensity score adjustment for clinical characteristics, the risk of death from any cause was higher in the HC group (adjusted hazard ratio [aHR], 1.83; 95% confidence interval [CI], 1.16-2.89; p = 0.009), but not in the HC+AZ group (aHR, 1.31; 95% CI, 0.80-2.15; p = 0.28). Both the propensity-score-adjusted risks of mechanical ventilation and death after mechanical ventilation were not significantly different in the HC group (aHR, 1.19; 95% CI, 0.78-1.82; p = 0.42 and aHR, 2.11; 95% CI, 0.96-4.62; p = 0.06, respectively) or in the HC+AZ group (aHR, 1.09; 95% CI, 0.72-1.66; p = 0.69 and aHR, 1.25; 95% CI, 0.59-2.68; p = 0.56, respectively) compared to the no HC group.
Conclusions: Among patients hospitalized with COVID-19, this retrospective study did not identify any significant reduction in mortality or in the need for mechanical ventilation with hydroxychloroquine treatment with or without azithromycin.
Funding: University of Virginia Strategic Investment Fund.
Keywords: COVID-19; SARS-CoV-2; azithromycin; hazard ratio; hydroxychloroquine; mortality; retrospective cohort; ventilation.
© 2020 Elsevier Inc.
Conflict of interest statement
J.A. is a co-founder of iVeena Holdings, iVeena Delivery Systems, and Inflammasome Therapeutics; he has received consultancy fees from Allergan, Biogen, Boehringer Ingelheim, Immunovant, Janssen, Olix Pharmaceuticals, Retinal Solutions, and Saksin LifeSciences, all for ophthalmic topics unrelated to COVID-19. J.A. is named as an inventor on a patent application filed by the University of Virginia relating to COVID-19 but unrelated to this work or to any ongoing COVID-19 clinical trials. S.S.S. has received research grants from Boehringer Ingelheim, Gilead Sciences, Portola Pharmaceuticals, and United Therapeutics, all for projects unrelated to COVID-19. J.W.H. has received consulting fees from Celgene Corporation unrelated to this work. The other authors declare no competing interests.
Figures
Update of
-
Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19.medRxiv [Preprint]. 2020 Apr 21:2020.04.16.20065920. doi: 10.1101/2020.04.16.20065920. medRxiv. 2020. Update in: Med. 2020 Dec 18;1(1):114-127.e3. doi: 10.1016/j.medj.2020.06.001. PMID: 32511622 Free PMC article. Updated. Preprint.
References
-
- Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. Published online March 20, 2020. - DOI - PMC - PubMed
-
- Food and Drug Administration . 2020. Fact sheet for health care providers: emergency use authorization (EUA) of hyroxychlroquine sulfate supplied from the strategic national stockpile for treatment of COVID-19 in certain hospitalized patients.https://www.fda.gov/media/136537/download
-
- Chorin E., Dai M., Shulman E., Wadhwani L., Bar-Cohen R., Barbhaiya C., Aizer A., Holmes D., Bernstein S., Spinelli M. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nat. Med. 2020 doi: 10.1038/s41591-020-0888-2. Published online April 24, 2020. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous