Aerosol risk with noninvasive respiratory support in patients with COVID-19
- PMID: 32838370
- PMCID: PMC7280651
- DOI: 10.1002/emp2.12152
Aerosol risk with noninvasive respiratory support in patients with COVID-19
Abstract
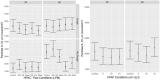
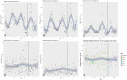
Objectives: This study evaluates aerosol production with high-flow nasal cannula (HFNC) and noninvasive positive pressure ventilation (NIPPV) compared to 6 L/min by low-flow nasal cannula.
Methods: Two healthy volunteers were randomized to control (6 L/min by low-flow nasal cannula), NIPPV, or HFNC using block randomization. NIPPV conditions were studied using continuous positive airway pressures of 5, 10, and 15 cm H2O with an FiO2 of 1.0 delivered via full-face mask. HFNC conditions included flow rates of 30 and 40 L/min with an FiO2 of 1.0 with and without coughing. HFNC and low-flow nasal cannula conditions were repeated with and without participants wearing a surgical mask. Six aerosol sizes (0.3, 1.0, 2.5, 5, and 10 µm) and total aerosol mass were measured at 2 and 6 ft from the participant's nasopharynx.
Results: There was no significant difference in aerosol production between either HFNC or NIPPV and control. There was also no significant difference with the use of a procedural mask over the HFNC. There was significant variation between the 2 participants, but in neither case was there a difference compared to control. There was an aerosol-time trend, but there does not appear to be a difference between either flow rate, pressure, or control. Furthermore, there was no accumulation of total aerosol particles over the total duration of the experiment in both HFNC and NIPPV conditions.
Conclusions: HFNC and NIPPV did not increase aerosol production compared to 6 L/min by low-flow nasal cannula in this experiment involving healthy volunteers.
© 2020 The Authors. JACEP Open published by Wiley Periodicals LLC on behalf of the American College of Emergency Physicians.
Conflict of interest statement
The authors report no conflicts of interest.Jarrod M. Mosier and David C. Miller conceived the study idea. Jarrod M. Mosier, David C. Miller, Paloma Beamer, Dean Billheimer, Vignesh Subbian designed the experiment. Jarrod M. Mosier and David C. Miller collected the data. Dean Billheimer performed the statistical analysis. All authors interpreted the results. Jarrod M. Mosier and David C. Miller drafted the initial manuscript and all authors contributed significantly to the revisions. Jarrod M. Mosier takes responsibility for the project as a whole.
Figures
References
-
- Hui DS, Chow BK, Lo T, et al. Exhaled air dispersion during high‐flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019;53:1802339. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

