Polydopamine-Mesoporous Silica Core-Shell Nanoparticles for Combined Photothermal Immunotherapy
- PMID: 32838525
- PMCID: PMC7942218
- DOI: 10.1021/acsami.0c10781
Polydopamine-Mesoporous Silica Core-Shell Nanoparticles for Combined Photothermal Immunotherapy
Abstract
Cancer immunotherapy involves a cascade of events that ultimately leads to cytotoxic immune cells effectively identifying and destroying cancer cells. Responsive nanomaterials, which enable spatiotemporal orchestration of various immunological events for mounting a highly potent and long-lasting antitumor immune response, are an attractive platform to overcome challenges associated with existing cancer immunotherapies. Here, we report a multifunctional near-infrared (NIR)-responsive core-shell nanoparticle, which enables (i) photothermal ablation of cancer cells for generating tumor-associated antigen (TAA) and (ii) triggered release of an immunomodulatory drug (gardiquimod) for starting a series of immunological events. The core of these nanostructures is composed of a polydopamine nanoparticle, which serves as a photothermal agent, and the shell is made of mesoporous silica, which serves as a drug carrier. We employed a phase-change material as a gatekeeper to achieve concurrent release of both TAA and adjuvant, thus efficiently activating the antigen-presenting cells. Photothermal immunotherapy enabled by these nanostructures resulted in regression of primary tumor and significantly improved inhibition of secondary tumor in a mouse melanoma model. These biocompatible, biodegradable, and NIR-responsive core-shell nanostructures simultaneously deliver payload and cause photothermal ablation of the cancer cells. Our results demonstrate potential of responsive nanomaterials in generating highly synergistic photothermal immunotherapeutic response.
Keywords: NIR-responsive drug delivery; cancer immunotherapy; mesoporous silica; photothermal therapy; polydopamine nanoparticles.
Conflict of interest statement
The authors declare no competing financial interests.
Supporting Information
Analysis of TGA data; Loading and release of LT680; Photothermal efficiency calculation; SEM image of as-prepared silica-coated PDA nanoparticles before removing the pore-template (CTAB); HRTEM image of PDA@mSiO2 nanoparticles and corresponding EDS elemental mapping for N and Si; Absorbance spectra of PDA and PDA@mSiO2 nanoparticles; TEM images of the PDA@mSiO2 nanoparticles before and after 10 minutes laser treatment;
Figures


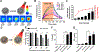
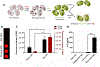


References
-
- Vesely MD; Kershaw MH; Schreiber RD; Smyth MJ, Natural Innate and Adaptive Immunity to Cancer. Annu. Rev. Immunol 2011, 29 (1), 235–271. - PubMed
-
- Dunn GP; Bruce AT; Ikeda H; Old LJ; Schreiber RD, Cancer Immunoediting: From Immunosurveillance to Tumor Escape. Nat. Immunol 2002, 3 (11), 991–998. - PubMed
-
- O’Donnell JS; Teng MWL; Smyth MJ, Cancer Immunoediting and Resistance to T Cell-Based Immunotherapy. Nat. Rev. Clin. Oncol 2018. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

