Differential processing and localization of human Nocturnin controls metabolism of mRNA and nicotinamide adenine dinucleotide cofactors
- PMID: 32839274
- PMCID: PMC7606674
- DOI: 10.1074/jbc.RA120.012618
Differential processing and localization of human Nocturnin controls metabolism of mRNA and nicotinamide adenine dinucleotide cofactors
Abstract
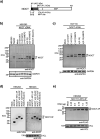
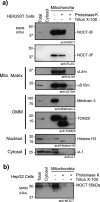
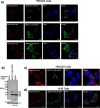
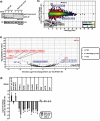
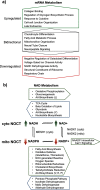
Nocturnin (NOCT) is a eukaryotic enzyme that belongs to a superfamily of exoribonucleases, endonucleases, and phosphatases. In this study, we analyze the expression, processing, localization, and cellular functions of human NOCT. We find that NOCT protein is differentially expressed and processed in a cell and tissue type-specific manner to control its localization to the cytoplasm or mitochondrial exterior or interior. The N terminus of NOCT is necessary and sufficient to confer import and processing in the mitochondria. We measured the impact of cytoplasmic NOCT on the transcriptome and observed that it affects mRNA levels of hundreds of genes that are significantly enriched in osteoblast, neuronal, and mitochondrial functions. Recent biochemical data indicate that NOCT dephosphorylates NADP(H) metabolites, and thus we measured the effect of NOCT on these cofactors in cells. We find that NOCT increases NAD(H) and decreases NADP(H) levels in a manner dependent on its intracellular localization. Collectively, our data indicate that NOCT can regulate levels of both mRNAs and NADP(H) cofactors in a manner specified by its location in cells.
Keywords: NOCT; Nocturnin; exoribonuclease; gene regulation; mRNA; mRNA decay; mitochondria; nicotinamide adenine dinucleotide; nicotinamide adenine dinucleotide (NAD); ribonuclease.
© 2020 Abshire et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures



Similar articles
-
The structure of human Nocturnin reveals a conserved ribonuclease domain that represses target transcript translation and abundance in cells.Nucleic Acids Res. 2018 Jul 6;46(12):6257-6270. doi: 10.1093/nar/gky412. Nucleic Acids Res. 2018. PMID: 29860338 Free PMC article.
-
A novel mouse model overexpressing Nocturnin results in decreased fat mass in male mice.J Cell Physiol. 2019 Nov;234(11):20228-20239. doi: 10.1002/jcp.28623. Epub 2019 Apr 5. J Cell Physiol. 2019. PMID: 30953371 Free PMC article.
-
Spatiotemporal regulation of NADP(H) phosphatase Nocturnin and its role in oxidative stress response.Proc Natl Acad Sci U S A. 2020 Jan 14;117(2):993-999. doi: 10.1073/pnas.1913712117. Epub 2019 Dec 26. Proc Natl Acad Sci U S A. 2020. PMID: 31879354 Free PMC article.
-
Homeostatic regulation of NAD(H) and NADP(H) in cells.Genes Dis. 2023 Oct 17;11(5):101146. doi: 10.1016/j.gendis.2023.101146. eCollection 2024 Sep. Genes Dis. 2023. PMID: 38988322 Free PMC article. Review.
-
Circadian control of Nocturnin and its regulatory role in health and disease.Chronobiol Int. 2023 Jul 3;40(7):970-981. doi: 10.1080/07420528.2023.2231081. Epub 2023 Jul 3. Chronobiol Int. 2023. PMID: 37400970 Review.
Cited by
-
Systematic identification and characterization of genes in the regulation and biogenesis of photosynthetic machinery.Cell. 2023 Dec 7;186(25):5638-5655.e25. doi: 10.1016/j.cell.2023.11.007. Cell. 2023. PMID: 38065083 Free PMC article.
-
Gene Characterization of Nocturnin Paralogues in Goldfish: Full Coding Sequences, Structure, Phylogeny and Tissue Expression.Int J Mol Sci. 2023 Dec 19;25(1):54. doi: 10.3390/ijms25010054. Int J Mol Sci. 2023. PMID: 38203224 Free PMC article.
-
How RNases Shape Mitochondrial Transcriptomes.Int J Mol Sci. 2022 May 30;23(11):6141. doi: 10.3390/ijms23116141. Int J Mol Sci. 2022. PMID: 35682820 Free PMC article. Review.
-
The Vsr-like protein FASTKD4 regulates the stability and polyadenylation of the MT-ND3 mRNA.Nucleic Acids Res. 2025 Feb 8;53(4):gkae1261. doi: 10.1093/nar/gkae1261. Nucleic Acids Res. 2025. PMID: 39727163 Free PMC article.
-
Oncometabolite 2-hydroxyglutarate suppresses basal protein levels of DNA polymerase beta that enhances alkylating agent and PARG inhibition induced cytotoxicity.DNA Repair (Amst). 2024 Aug;140:103700. doi: 10.1016/j.dnarep.2024.103700. Epub 2024 Jun 4. DNA Repair (Amst). 2024. PMID: 38897003 Free PMC article.
References
-
- Abshire E. T., Chasseur J., Bohn J. A., Del Rizzo P. A., Freddolino P. L., Goldstrohm A. C., and Trievel R. C. (2018) The structure of human Nocturnin reveals a conserved ribonuclease domain that represses target transcript translation and abundance in cells. Nucleic Acids Res. 46, 6257–6270 10.1093/nar/gky412 - DOI - PMC - PubMed
-
- Blanco A. M., Gomez-Boronat M., Madera D., Valenciano A. I., Alonso-Gomez A. L., and Delgado M. J. (2017) First evidence of nocturnin in fish: two isoforms in goldfish differentially regulated by feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 314, R304–R312 10.1152/ajpregu.00241.2017 - DOI - PubMed
-
- Green C. B., Douris N., Kojima S., Strayer C. A., Fogerty J., Lourim D., Keller S. R., and Besharse J. C. (2007) Loss of Nocturnin, a circadian deadenylase, confers resistance to hepatic steatosis and diet-induced obesity. Proc. Natl. Acad. Sci. U. S. A. 104, 9888–9893 10.1073/pnas.0702448104 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

