Prolonged QT Interval in SARS-CoV-2 Infection: Prevalence and Prognosis
- PMID: 32839385
- PMCID: PMC7563186
- DOI: 10.3390/jcm9092712
Prolonged QT Interval in SARS-CoV-2 Infection: Prevalence and Prognosis
Abstract
Background: The prognostic value of a prolonged QT interval in SARS-Cov2 infection is not well known.
Objective: To determine whether the presence of a prolonged QT on admission is an independent factor for mortality in SARS-Cov2 hospitalized patients.
Methods: Single-center cohort of 623 consecutive patients with positive polymerase-chain-reaction test (PCR) to SARS Cov2, recruited from 27 February to 7 April 2020. An electrocardiogram was taken on these patients within the first 48 h after diagnosis and before the administration of any medication with a known effect on QT interval. A prolonged QT interval was defined as a corrected QT (QTc) interval >480 milliseconds. Patients were followed up with until 10 May 2020.
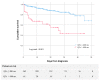
Results: Sixty-one patients (9.8%) had prolonged QTc and only 3.2% had a baseline QTc > 500 milliseconds. Patients with prolonged QTc were older, had more comorbidities, and higher levels of immune-inflammatory markers. There were no episodes of ventricular tachycardia or ventricular fibrillation during hospitalization. All-cause death was higher in patients with prolonged QTc (41.0% vs. 8.7%, p < 0.001, multivariable HR 2.68 (1.58-4.55), p < 0.001).
Conclusions: Almost 10% of patients with COVID-19 infection have a prolonged QTc interval on admission. A prolonged QTc was independently associated with a higher mortality even after adjustment for age, comorbidities, and treatment with hydroxychloroquine and azithromycin. An electrocardiogram should be included on admission to identify high-risk SARS-CoV-2 patients.
Keywords: COVID-19; QT interval; azithromycin; death; hydroxychloroquine; prognosis.
Conflict of interest statement
N.F. reports grants and consultancy fees from Novartis, Bayer, Rovi, and AstraZeneca; J.M. reports grants and consultancy fees from AstraZeneca, Sanofi, Shire, Gilead, Daichii-Sankyo, Genincode, and Ferrer. The other authors declare no conflict of interest.
Figures
References
-
- Saleh M., Gabriels J., Chang D., Kim B.S., Mansoor A., Mahmood E., Makker P., Ismail H., Goldner B., Willner J., et al. The Effect of Chloroquine, Hydroxychloroquine and Azithromycin on the Corrected QT Interval in Patients with SARS-CoV-2 Infection. Circ. Arrhythm. Electrophysiol. 2020 doi: 10.1161/CIRCEP.120.008662. - DOI - PMC - PubMed
-
- Mercuro N.J., Yen C.F., Shim D.J., Maher T.R., McCoy C.M., Zimetbaum P.J., Gold H.S. Risk of QT Interval Prolongation Associated with Use of Hydroxychloroquine with or without Concomitant Azithromycin among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1834. - DOI - PMC - PubMed
-
- Ramireddy A., Chugh H., Reinier K., Ebinger J., Park E., Thompson M., Cingolani E., Cheng S., Marban E., Albert C.M., et al. Experience With Hydroxychloroquine and Azithromycin in the Coronavirus Disease 2019 Pandemic: Implications for QT Interval Monitoring. J. Am. Heart Assoc. 2020;9:e017144. doi: 10.1161/JAHA.120.017144. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous