Lipid Players of Cellular Senescence
- PMID: 32839400
- PMCID: PMC7570155
- DOI: 10.3390/metabo10090339
Lipid Players of Cellular Senescence
Abstract
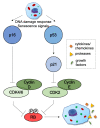
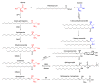
Lipids are emerging as key players of senescence. Here, we review the exciting new findings on the diverse roles of lipids in cellular senescence, most of which are enabled by the advancements in omics approaches. Senescence is a cellular process in which the cell undergoes growth arrest while retaining metabolic activity. At the organismal level, senescence contributes to organismal aging and has been linked to numerous diseases. Current research has documented that senescent cells exhibit global alterations in lipid composition, leading to extensive morphological changes through membrane remodeling. Moreover, senescent cells adopt a secretory phenotype, releasing various components to their environment that can affect the surrounding tissue and induce an inflammatory response. All of these changes are membrane and, thus, lipid related. Our work, and that of others, has revealed that fatty acids, sphingolipids, and glycerolipids are involved in the initiation and maintenance of senescence and its associated inflammatory components. These studies opened up an exciting frontier to investigate the deeper mechanistic understanding of the regulation and function of these lipids in senescence. In this review, we will provide a comprehensive snapshot of the current state of the field and share our enthusiasm for the prospect of potential lipid-related protein targets for small-molecule therapy in pathologies involving senescence and its related inflammatory phenotypes.
Keywords: fatty acids; glycerolipids; lipids; senescence; sphingolipids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Lipids as Regulators of Cellular Senescence.Front Physiol. 2022 Mar 4;13:796850. doi: 10.3389/fphys.2022.796850. eCollection 2022. Front Physiol. 2022. PMID: 35370799 Free PMC article. Review.
-
Lipids and lipid metabolism in cellular senescence: Emerging targets for age-related diseases.Ageing Res Rev. 2024 Jun;97:102294. doi: 10.1016/j.arr.2024.102294. Epub 2024 Apr 5. Ageing Res Rev. 2024. PMID: 38583577 Review.
-
The emerging role of senescent cells in tissue homeostasis and pathophysiology.Pathobiol Aging Age Relat Dis. 2015 May 19;5:27743. doi: 10.3402/pba.v5.27743. eCollection 2015. Pathobiol Aging Age Relat Dis. 2015. PMID: 25994420 Free PMC article.
-
Developing senescence to remodel the embryo.Commun Integr Biol. 2014 Oct 31;7(5):e970969. doi: 10.4161/cib.29098. eCollection 2014 Oct. Commun Integr Biol. 2014. PMID: 26842300 Free PMC article.
-
Biology of extracellular vesicles secreted from senescent cells as senescence-associated secretory phenotype factors.Geriatr Gerontol Int. 2020 Jun;20(6):539-546. doi: 10.1111/ggi.13928. Epub 2020 May 1. Geriatr Gerontol Int. 2020. PMID: 32358923 Review.
Cited by
-
Vascular smooth muscle cell senescence accelerates medin aggregation via small extracellular vesicle secretion and extracellular matrix reorganization.Aging Cell. 2023 Feb;22(2):e13746. doi: 10.1111/acel.13746. Epub 2022 Nov 25. Aging Cell. 2023. PMID: 36433666 Free PMC article.
-
Lipid accumulation drives cellular senescence in dopaminergic neurons.Aging (Albany NY). 2024 Jul 19;16(14):11128-11133. doi: 10.18632/aging.206030. Epub 2024 Jul 19. Aging (Albany NY). 2024. PMID: 39033779 Free PMC article. Review.
-
Nutritional components as mitigators of cellular senescence in organismal aging: a comprehensive review.Food Sci Biotechnol. 2022 Jun 18;31(9):1089-1109. doi: 10.1007/s10068-022-01114-y. eCollection 2022 Aug. Food Sci Biotechnol. 2022. PMID: 35756719 Free PMC article. Review.
-
Potential of Naturally Derived Compounds in Telomerase and Telomere Modulation in Skin Senescence and Aging.Int J Mol Sci. 2021 Jun 15;22(12):6381. doi: 10.3390/ijms22126381. Int J Mol Sci. 2021. PMID: 34203694 Free PMC article. Review.
-
Tangential flow filtration-facilitated purification of human red blood cell membrane fragments and its preferential use in removing unencapsulated material from resealed red blood cell ghosts compared to centrifugation.Biotechnol Prog. 2024 Nov-Dec;40(6):e3501. doi: 10.1002/btpr.3501. Epub 2024 Jul 30. Biotechnol Prog. 2024. PMID: 39076022 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

