In Vivo TSPO Signal and Neuroinflammation in Alzheimer's Disease
- PMID: 32839410
- PMCID: PMC7565089
- DOI: 10.3390/cells9091941
In Vivo TSPO Signal and Neuroinflammation in Alzheimer's Disease
Abstract
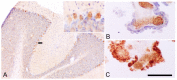
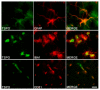
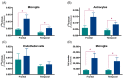
In the last decade, positron emission tomography (PET) and single-photon emission computed tomography (SPECT) in in vivo imaging has attempted to demonstrate the presence of neuroinflammatory reactions by measuring the 18 kDa translocator protein (TSPO) expression in many diseases of the central nervous system. We focus on two pathological conditions for which neuropathological studies have shown the presence of neuroinflammation, which translates in opposite in vivo expression of TSPO. Alzheimer's disease has been the most widely assessed with more than forty preclinical and clinical studies, showing overall that TSPO is upregulated in this condition, despite differences in the topography of this increase, its time-course and the associated cell types. In the case of schizophrenia, a reduction of TSPO has instead been observed, though the evidence remains scarce and contradictory. This review focuses on the key characteristics of TSPO as a biomarker of neuroinflammation in vivo, namely, on the cellular origin of the variations in its expression, on its possible biological/pathological role and on its variations across disease phases.
Keywords: Alzheimer’s disease; TSPO; astrocytes; microglia; schizophrenia.
Conflict of interest statement
The author(s) declared no conflicts of interest
Figures



References
-
- Brosseron F., Traschutz A., Widmann C.N., Kummer M.P., Tacik P., Santarelli F., Jessen F., Heneka M.T. Characterization and clinical use of inflammatory cerebrospinal fluid protein markers in Alzheimer’s disease. Alzheimer’s Res. Ther. 2018;10:25. doi: 10.1186/s13195-018-0353-3. - DOI - PMC - PubMed
-
- Lai K.S.P., Liu C.S., Rau A., Lanctot K.L., Kohler C.A., Pakosh M., Carvalho A.F., Herrmann N. Peripheral inflammatory markers in Alzheimer’s disease: A systematic review and meta-analysis of 175 studies. J. Neurol. Neurosurg. Psychiatry. 2017;88:876–882. doi: 10.1136/jnnp-2017-316201. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

