Anti-Metastatic Activity of an Anti-EGFR Monoclonal Antibody against Metastatic Colorectal Cancer with KRAS p.G13D Mutation
- PMID: 32839411
- PMCID: PMC7504481
- DOI: 10.3390/ijms21176037
Anti-Metastatic Activity of an Anti-EGFR Monoclonal Antibody against Metastatic Colorectal Cancer with KRAS p.G13D Mutation
Abstract
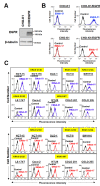
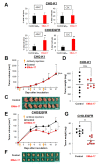
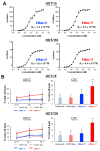
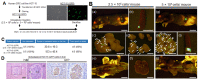
The now clinically-used anti-epidermal growth factor receptor (EGFR) monoclonal antibodies have demonstrated significant efficacy only in patients with metastatic colorectal cancer (mCRC), with wild-type Kirsten rat sarcoma viral oncogene homolog (KRAS). However, no effective treatments for patients with mCRC with KRAS mutated tumors have been approved yet. Therefore, a new strategy for targeting mCRC with KRAS mutated tumors is desired. In the present study, we examined the anti-tumor activities of a novel anti-EGFR monoclonal antibody, EMab-17 (mouse IgG2a, kappa), in colorectal cancer (CRC) cells with the KRAS p.G13D mutation. This antibody recognized endogenous EGRF in CRC cells with or without KRAS mutations, and showed a high sensitivity for CRC cells in flow cytometry, indicating that EMab-17 possesses a high binding affinity to the endogenous EGFR. In vitro experiments showed that EMab-17 exhibited antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity activities against CRC cells. In vivo analysis revealed that EMab-17 inhibited the metastases of HCT-15 and HCT-116 cells in the livers of nude mouse metastatic models, unlike the anti-EGFR monoclonal antibody EMab-51 of subtype mouse IgG1. In conclusion, EMab-17 may be useful in an antibody-based therapy against mCRC with the KRAS p.G13D mutation.
Keywords: antibody-dependent cell cytotoxicity; colorectal cancer; complement-dependent cytotoxicity; epidermal growth factor receptor; metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Meta-analysis comparing the efficacy of anti-EGFR monoclonal antibody therapy between KRAS G13D and other KRAS mutant metastatic colorectal cancer tumours.Eur J Cancer. 2016 Mar;55:122-30. doi: 10.1016/j.ejca.2015.11.025. Epub 2016 Jan 23. Eur J Cancer. 2016. PMID: 26812186
-
Promising Therapeutic Efficacy of GC1118, an Anti-EGFR Antibody, against KRAS Mutation-Driven Colorectal Cancer Patient-Derived Xenografts.Int J Mol Sci. 2019 Nov 24;20(23):5894. doi: 10.3390/ijms20235894. Int J Mol Sci. 2019. PMID: 31771279 Free PMC article.
-
AMPK activation overcomes anti-EGFR antibody resistance induced by KRAS mutation in colorectal cancer.Cell Commun Signal. 2020 Jul 23;18(1):115. doi: 10.1186/s12964-020-00584-z. Cell Commun Signal. 2020. PMID: 32703218 Free PMC article.
-
4-Acetyl-Antroquinonol B Improves the Sensitization of Cetuximab on Both Kras Mutant and Wild Type Colorectal Cancer by Modulating the Expression of Ras/Raf/miR-193a-3p Signaling Axis.Int J Mol Sci. 2021 Jul 14;22(14):7508. doi: 10.3390/ijms22147508. Int J Mol Sci. 2021. PMID: 34299137 Free PMC article.
-
Anti-epidermal growth factor receptor monoclonal antibodies in metastatic colorectal cancer: a meta-analysis.World J Gastroenterol. 2015 Apr 14;21(14):4365-72. doi: 10.3748/wjg.v21.i14.4365. World J Gastroenterol. 2015. PMID: 25892888 Free PMC article. Review.
Cited by
-
Defucosylated mouse‑dog chimeric anti‑HER2 monoclonal antibody exerts antitumor activities in mouse xenograft models of canine tumors.Oncol Rep. 2022 Sep;48(3):154. doi: 10.3892/or.2022.8366. Epub 2022 Jul 20. Oncol Rep. 2022. PMID: 35856438 Free PMC article.
-
Tumour Immunotherapy and Applications of Immunological Products: A Review of Literature.J Immunol Res. 2024 Oct 24;2024:8481761. doi: 10.1155/2024/8481761. eCollection 2024. J Immunol Res. 2024. PMID: 39483536 Free PMC article. Review.
-
Current Targeted Therapy for Metastatic Colorectal Cancer.Int J Mol Sci. 2023 Jan 15;24(2):1702. doi: 10.3390/ijms24021702. Int J Mol Sci. 2023. PMID: 36675216 Free PMC article. Review.
-
Defucosylated Mouse-Dog Chimeric Anti-EGFR Antibody Exerts Antitumor Activities in Mouse Xenograft Models of Canine Tumors.Cells. 2021 Dec 20;10(12):3599. doi: 10.3390/cells10123599. Cells. 2021. PMID: 34944112 Free PMC article.
-
A Defucosylated Anti-EpCAM Monoclonal Antibody (EpMab-37-mG2a-f) Exerts Antitumor Activity in Xenograft Model.Antibodies (Basel). 2022 Nov 24;11(4):74. doi: 10.3390/antib11040074. Antibodies (Basel). 2022. PMID: 36546899 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- 19cm0106213h0004/Japan Agency for Medical Research and Development
- JP19am0401013/Japan Agency for Medical Research and Development
- JP19am0101078/Japan Agency for Medical Research and Development
- JP19ae0101028/Japan Agency for Medical Research and Development
- 18K08693/Japan Society for the Promotion of Science
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

