Gut bacteria-derived peptidoglycan induces a metabolic syndrome-like phenotype via NF-κB-dependent insulin/PI3K signaling reduction in Drosophila renal system
- PMID: 32839462
- PMCID: PMC7445169
- DOI: 10.1038/s41598-020-70455-7
Gut bacteria-derived peptidoglycan induces a metabolic syndrome-like phenotype via NF-κB-dependent insulin/PI3K signaling reduction in Drosophila renal system
Abstract
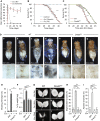
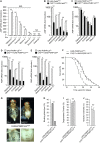
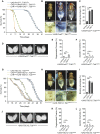
Although microbiome-host interactions are usual at steady state, gut microbiota dysbiosis can unbalance the physiological and behavioral parameters of the host, mostly via yet not understood mechanisms. Using the Drosophila model, we investigated the consequences of a gut chronic dysbiosis on the host physiology. Our results show that adult flies chronically infected with the non-pathogenic Erwinia carotorova caotovora bacteria displayed organ degeneration resembling wasting-like phenotypes reminiscent of Metabolic Syndrome associated pathologies. Genetic manipulations demonstrate that a local reduction of insulin signaling consecutive to a peptidoglycan-dependent NF-κB activation in the excretory system of the flies is responsible for several of the observed phenotypes. This work establishes a functional crosstalk between bacteria-derived peptidoglycan and the immune NF-κB cascade that contributes to the onset of metabolic disorders by reducing insulin signal transduction. Giving the high degree of evolutionary conservation of the mechanisms and pathways involved, this study is likely to provide a helpful model to elucidate the contribution of altered intestinal microbiota in triggering human chronic kidney diseases.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Cytosolic and Secreted Peptidoglycan-Degrading Enzymes in Drosophila Respectively Control Local and Systemic Immune Responses to Microbiota.Cell Host Microbe. 2018 Feb 14;23(2):215-228.e4. doi: 10.1016/j.chom.2017.12.007. Epub 2018 Feb 1. Cell Host Microbe. 2018. PMID: 29398649
-
Bacterial peptidoglycan serves as a critical modulator of the gut-immune-brain axis in Drosophila.Brain Behav Immun. 2024 Jul;119:878-897. doi: 10.1016/j.bbi.2024.05.009. Epub 2024 May 6. Brain Behav Immun. 2024. PMID: 38710338
-
Gut-derived peptidoglycan remotely inhibits bacteria dependent activation of SREBP by Drosophila adipocytes.PLoS Genet. 2022 Mar 4;18(3):e1010098. doi: 10.1371/journal.pgen.1010098. eCollection 2022 Mar. PLoS Genet. 2022. PMID: 35245295 Free PMC article.
-
[Gut microbiota and immune crosstalk in metabolic disease].Biol Aujourdhui. 2017;211(1):1-18. doi: 10.1051/jbio/2017008. Epub 2017 Jul 6. Biol Aujourdhui. 2017. PMID: 28682223 Review. French.
-
Bacteria sensing mechanisms in Drosophila gut: Local and systemic consequences.Dev Comp Immunol. 2016 Nov;64:11-21. doi: 10.1016/j.dci.2016.01.001. Epub 2016 Jan 8. Dev Comp Immunol. 2016. PMID: 26778296 Review.
Cited by
-
Gut microbiota associations with metabolic syndrome and relevance of its study in pediatric subjects.Gut Microbes. 2021 Jan-Dec;13(1):1960135. doi: 10.1080/19490976.2021.1960135. Gut Microbes. 2021. PMID: 34491882 Free PMC article. Review.
-
Steroid hormone regulation of innate immunity in Drosophila melanogaster.PLoS Genet. 2023 Jun 15;19(6):e1010782. doi: 10.1371/journal.pgen.1010782. eCollection 2023 Jun. PLoS Genet. 2023. PMID: 37319172 Free PMC article. Review.
-
Translocation of gut bacteria promotes tumor-associated mortality by inducing immune-activated renal damage.EMBO J. 2025 Jul;44(13):3586-3613. doi: 10.1038/s44318-025-00458-5. Epub 2025 May 22. EMBO J. 2025. PMID: 40404992 Free PMC article.
-
Investigating local and systemic intestinal signalling in health and disease with Drosophila.Dis Model Mech. 2022 Mar 1;15(3):dmm049332. doi: 10.1242/dmm.049332. Epub 2022 Mar 28. Dis Model Mech. 2022. PMID: 35344037 Free PMC article. Review.
-
metaGEM: reconstruction of genome scale metabolic models directly from metagenomes.Nucleic Acids Res. 2021 Dec 2;49(21):e126. doi: 10.1093/nar/gkab815. Nucleic Acids Res. 2021. PMID: 34614189 Free PMC article.
References
-
- Cani PD. Microbiota and metabolites in metabolic diseases. Nat. Rev. Endocrinol. 2019;15:69–70. - PubMed
-
- Tilg H, Zmora N, Adolph TE, Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020;20:40–54. - PubMed
-
- Singh AK, Kari JA. Metabolic syndrome and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2013;22:198–203. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

